Abstract
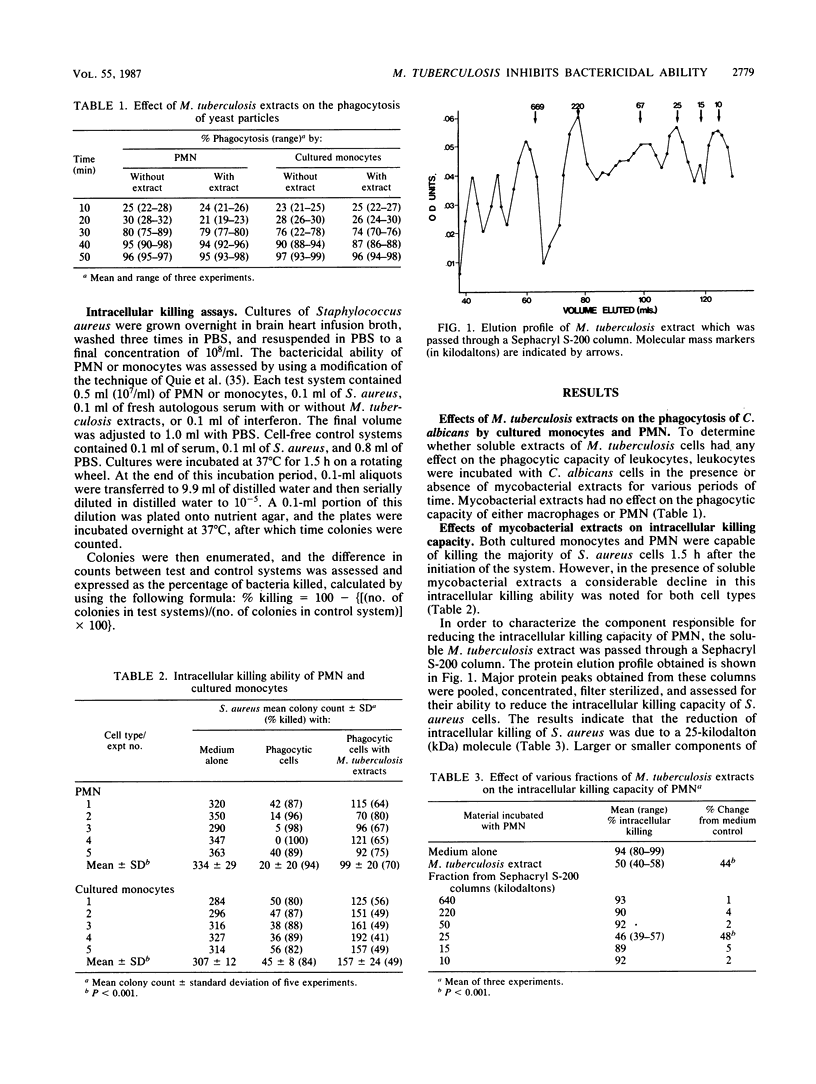
In this study we examined the effects of Mycobacterium tuberculosis cell extracts on the phagocytic activity of polymorphonuclear leukocytes and cultured peripheral blood monocytes. M. tuberculosis cell extracts were fractionated on Sephacryl S-200 columns, and a 25-kilodalton glycolipoprotein was shown to inhibit the intracellular killing ability of these leukocytes but had no effect on their phagocytic potential. This same fraction inhibited fusion of phagosomes with lysosomes, as assessed by noting the transfer of acridine orange from lysosomes to phagosomes. This fraction was shown to have a maximal inhibitory effect when it was in the form of an intact carbohydrate-lipid-protein complex. Gamma interferon (IFN-gamma), but not IFN-alpha, reversed the inhibitory effect of the mycobacterial component on bactericidal activity and on fusion of phagosomes and lysosomes. Thus, this 25-kilodalton fraction of M. tuberculosis cell extract may be important in protecting organisms against phagocytic degradation, an effect which can be reversed by IFN-gamma.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. Lysosomes and disease. Sci Am. 1967 Nov;217(5):62–72. doi: 10.1038/scientificamerican1167-62. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axline S. G., Cohn Z. A. In vitro induction of lysosomal enzymes by phagocytosis. J Exp Med. 1970 Jun 1;131(6):1239–1260. doi: 10.1084/jem.131.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N., Nash T. W., Horwitz M. A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986 Oct 15;137(8):2662–2669. [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Brown C. A., Draper P., Hart P. D. Mycobacteria and lysosomes: a paradox. Nature. 1969 Feb 15;221(5181):658–660. doi: 10.1038/221658a0. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A., Hirsch J. G., Fedorko M. E. The in vitro differentiation of mononuclear phagocytes. IV. The ultrastructure of macrophage differentiation in the peritoneal cavity and in culture. J Exp Med. 1966 Apr 1;123(4):747–756. doi: 10.1084/jem.123.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree I. A., Beck J. S. The influence of killed Mycobacterium leprae and other mycobacteria on opsonized yeast phagocytosis. Clin Exp Immunol. 1986 Apr;64(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Hedegaard H. B., Zlotnik A., Gangadharam P. R., Johnston R. B., Jr, Pabst M. J. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol. 1986 Mar 1;136(5):1820–1827. [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisow M. J., Beaven G. H., Hart P. D., Young M. R. Site of action of a polyanion inhibitor of phagosome-lysosome fusion in cultured macrophages. Exp Cell Res. 1980 Mar;126(1):159–165. doi: 10.1016/0014-4827(80)90481-4. [DOI] [PubMed] [Google Scholar]
- Gordon A. H., Hart P. D., Young M. R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980 Jul 3;286(5768):79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., Swendsen C. L., Fiscus J., Miranti C. Fluorescent markers for studying phagosome-lysosome fusion. J Leukoc Biol. 1984 Sep;36(3):273–292. doi: 10.1002/jlb.36.3.273. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Vatter A. E., Fiscus J. Polyanionic agents as inhibitors of phagosome-lysosome fusion in cultured macrophages: evolution of an alternative interpretation. J Leukoc Biol. 1987 Feb;41(2):111–121. doi: 10.1002/jlb.41.2.111. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Vatter A. E., Fiscus J. Polyanionic agents do not inhibit phagosome-lysosome fusion in cultured macrophages. J Leukoc Biol. 1987 Feb;41(2):122–129. doi: 10.1002/jlb.41.2.122. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A. Strain virulence and the lysosomal response in macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1974 Oct;10(4):742–746. doi: 10.1128/iai.10.4.742-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie D. B., Jackett P. S., Ratcliffe N. A. Mycobacterium microti may protect itself from intracellular destruction by releasing cyclic AMP into phagosomes. Nature. 1975 Apr 17;254(5501):600–602. doi: 10.1038/254600a0. [DOI] [PubMed] [Google Scholar]
- McCarthy C. Synthesis and release of sulfolipid by Mycobacterium avium during growth andcell division. Infect Immun. 1976 Nov;14(5):1241–1252. doi: 10.1128/iai.14.5.1241-1252.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvik Q. N., Leake E. S., Wright M. J. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am Rev Respir Dis. 1984 Feb;129(2):322–328. [PubMed] [Google Scholar]
- Nacy C. A., James S. L., Benjamin W. R., Farrar J. J., Hockmeyer W. T., Meltzer M. S. Activation of macrophages for microbicidal and tumoricidal effector functions by soluble factors from EL-4, a continuous T cell line. Infect Immun. 1983 May;40(2):820–824. doi: 10.1128/iai.40.2.820-824.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn J. Y., Shadduck J. A. Role of antibody and complement in the control of Encephalitozoon cuniculi infections by rabbit macrophages. Infect Immun. 1980 Mar;27(3):995–1002. doi: 10.1128/iai.27.3.995-1002.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owhashi M., Horii Y., Ishii A. Eosinophil chemotactic factor in schistosome eggs: a comparative study of eosinophil chemotactic factors in the eggs of Schistosoma japonicum and S. mansoni in vitro. Am J Trop Med Hyg. 1983 Mar;32(2):359–366. doi: 10.4269/ajtmh.1983.32.359. [DOI] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Wadee A. A., Sher R., Rabson A. R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980 Sep;125(3):1380–1386. [PubMed] [Google Scholar]
- Weidner E. Interactions between Encephalitozoon cuniculi and macrophages. Parasitophorous vacuole growth and the absence of lysosomal fusion. Z Parasitenkd. 1975 Aug 21;47(1):1–9. doi: 10.1007/BF00418060. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Goldstein I., Hoffstein S. Prostaglandins and the modulation by cyclic nucleotides of lysosomal enzyme release. Adv Prostaglandin Thromboxane Res. 1976;2:803–814. [PubMed] [Google Scholar]
- Wheeler P. R., Gregory D. Superoxide dismutase, peroxidatic activity and catalase in Mycobacterium leprae purified from armadillo liver. J Gen Microbiol. 1980 Dec;121(2):457–464. doi: 10.1099/00221287-121-2-457. [DOI] [PubMed] [Google Scholar]


