Abstract
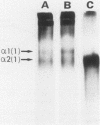
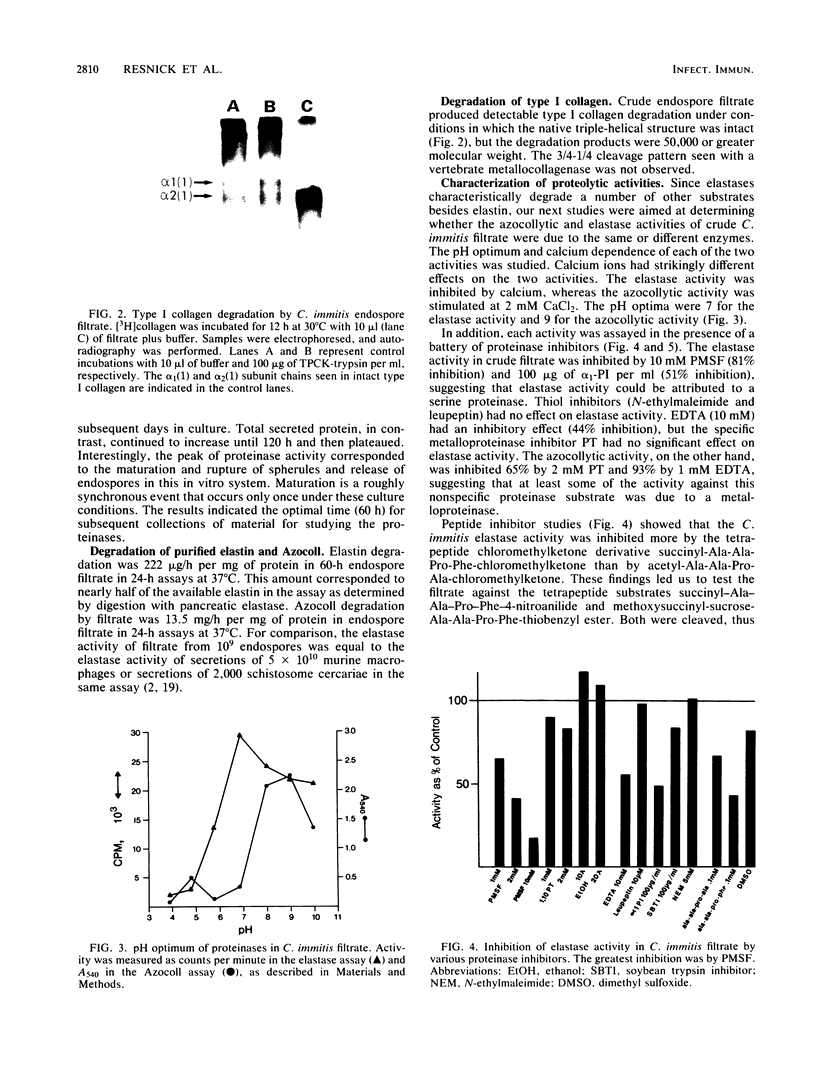
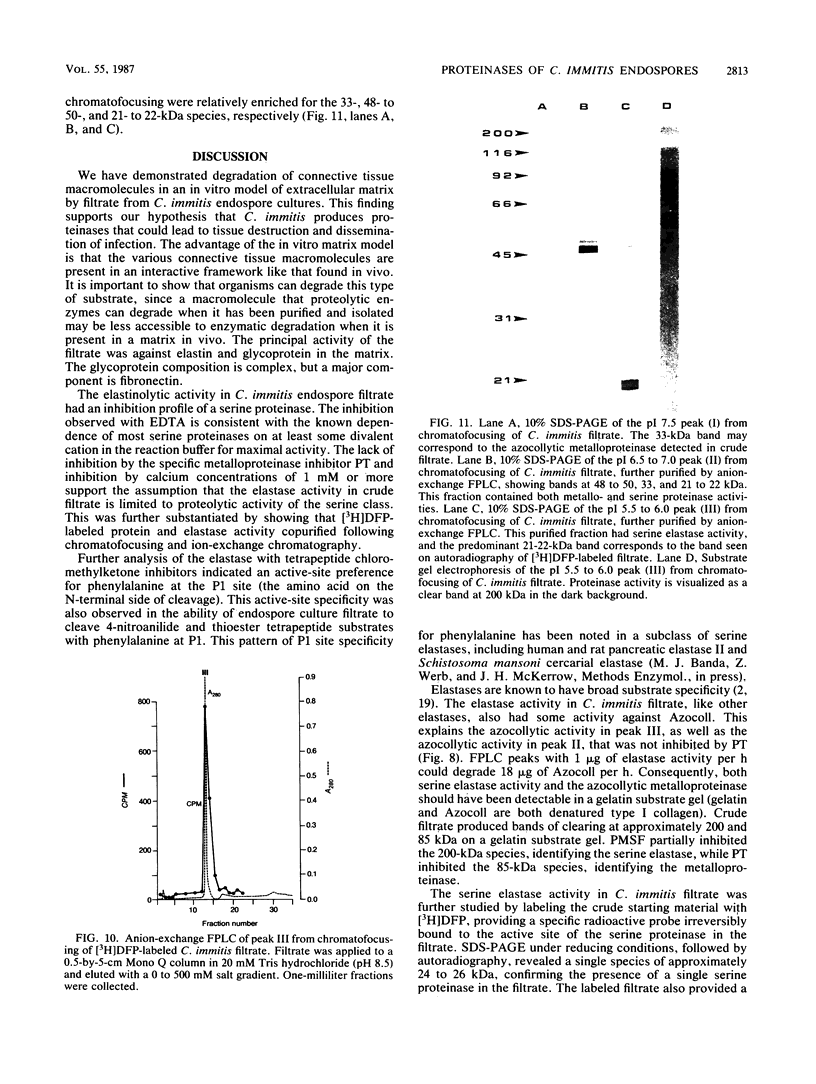
Coccidioides immitis is the causative agent of coccidioidomycosis (valley fever), a potentially disseminated fungal disease. We hypothesized that proteinases are expressed by the parasitic life cycle of C. immitis and that they might play an important role in the pathogenesis of coccidioidomycosis by facilitating spherule rupture, endospore dissemination, and tissue invasion and destruction. Filtrate from cultures of the parasitic life cycle of C. immitis was therefore assayed for proteolytic activity at neutral pH. The filtrate degraded 68% of a radiolabeled model of an elastin-rich extracellular matrix. The principal activity was against elastin and glycoprotein in the matrix. Degradation of purified elastin by filtrate was 222 micrograms/h per mg of filtrate protein at 37 degrees C. Denatured type I collagen (Azocoll) degradation was 13.5 mg/h per mg of filtrate protein at 37 degrees C. Proteinase activity peaked at 60 h of culture, correlating with release of endospores from mature spherules in the in vitro culture system. Elastase activity was attributed to a serine proteinase which exhibited an active-site preference for phenylalanine at the P1 site. The subunit molecular mass of the elastase determined by [3H]diisopropylfluorophosphate labeling was approximately 25 kilodaltons. Inhibition of the azocollytic activity of crude filtrate by 2 mM 1,10-phenanthroline and 10 mM EDTA, and stimulation by 2 mM CaCl2, suggested that a metalloproteinase was also present. Gelatin substrate gel electrophoresis with and without inhibitors confirmed that two proteinases were expressed, and they were separated by fast protein liquid chromatography.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banda M. J., Clark E. J., Werb Z. Limited proteolysis by macrophage elastase inactivates human alpha 1-proteinase inhibitor. J Exp Med. 1980 Dec 1;152(6):1563–1570. doi: 10.1084/jem.152.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo M. J., Nakajima K., Zimmerman M., Powers J. C. Sensitive substrates for human leukocyte and porcine pancreatic elastase: a study of the merits of various chromophoric and fluorogenic leaving groups in assays for serine proteases. Anal Biochem. 1979 Oct 15;99(1):53–64. doi: 10.1016/0003-2697(79)90043-5. [DOI] [PubMed] [Google Scholar]
- Drutz D. J., Catanzaro A. Coccidioidomycosis. Part I. Am Rev Respir Dis. 1978 Mar;117(3):559–585. doi: 10.1164/arrd.1978.117.3.559. [DOI] [PubMed] [Google Scholar]
- Gisslow M. T., McBride B. C. A rapid sensitive collagenase assay. Anal Biochem. 1975 Sep;68(1):70–78. doi: 10.1016/0003-2697(75)90680-6. [DOI] [PubMed] [Google Scholar]
- Heck L. W., Morihara K., McRae W. B., Miller E. J. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect Immun. 1986 Jan;51(1):115–118. doi: 10.1128/iai.51.1.115-118.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hotez P. J., Trang N. L., McKerrow J. H., Cerami A. Isolation and characterization of a proteolytic enzyme from the adult hookworm Ancylostoma caninum. J Biol Chem. 1985 Jun 25;260(12):7343–7348. [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Davidson J. M., Rennard S., Szapiel S., Gadek J. E., Crystal R. G. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science. 1981 May 22;212(4497):925–927. doi: 10.1126/science.7233186. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Scott-Burden T., Gevers W. Glycoprotein, elastin, and collagen secretion by rat smooth muscle cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):353–357. doi: 10.1073/pnas.76.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene W. E., Jeong K. H., McKerrow J. H., Werb Z. Degradation of extracellular matrix by larvae of Schistosoma mansoni. II. Degradation by newly transformed and developing schistosomula. Lab Invest. 1983 Aug;49(2):201–207. [PubMed] [Google Scholar]
- LEVINE H. B., COBB J. M., SMITH C. E. Immunity to coccidioi-domycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960 Apr;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist R. N., Senft A. W., Petitt M., McKerrow J. Schistosoma mansoni: purification and characterization of the major acidic proteinase from adult worms. Exp Parasitol. 1986 Jun;61(3):398–404. doi: 10.1016/0014-4894(86)90196-7. [DOI] [PubMed] [Google Scholar]
- Lupan D. M., Nziramasanga P. Collagenolytic activity of Coccidioides immitis. Infect Immun. 1986 Jan;51(1):360–361. doi: 10.1128/iai.51.1.360-361.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerrow J. H., Keene W. E., Jeong K. H., Werb Z. Degradation of extracellular matrix by larvae of Schistosoma mansoni. I. Degradation by cercariae as a model for initial parasite invasion of host. Lab Invest. 1983 Aug;49(2):195–200. [PubMed] [Google Scholar]
- McKerrow J. H., Pino-Heiss S., Lindquist R., Werb Z. Purification and characterization of an elastinolytic proteinase secreted by cercariae of Schistosoma mansoni. J Biol Chem. 1985 Mar 25;260(6):3703–3707. [PubMed] [Google Scholar]
- Powers J. C., Tuhy P. M. Active-site specific inhibitors of elastase. Biochemistry. 1973 Nov 6;12(23):4767–4774. doi: 10.1021/bi00747a032. [DOI] [PubMed] [Google Scholar]
- Rippon J. W., Varadi D. P. The elastases of pathogenic fungi and actinomycetes. J Invest Dermatol. 1968 Jan;50(1):54–58. doi: 10.1038/jid.1968.8. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980 Oct;66(4):859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Banda M. J., Jones P. A. Degradation of connective tissue matrices by macrophages. I. Proteolysis of elastin, glycoproteins, and collagen by proteinases isolated from macrophages. J Exp Med. 1980 Nov 1;152(5):1340–1357. doi: 10.1084/jem.152.5.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]