Abstract
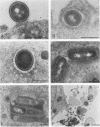
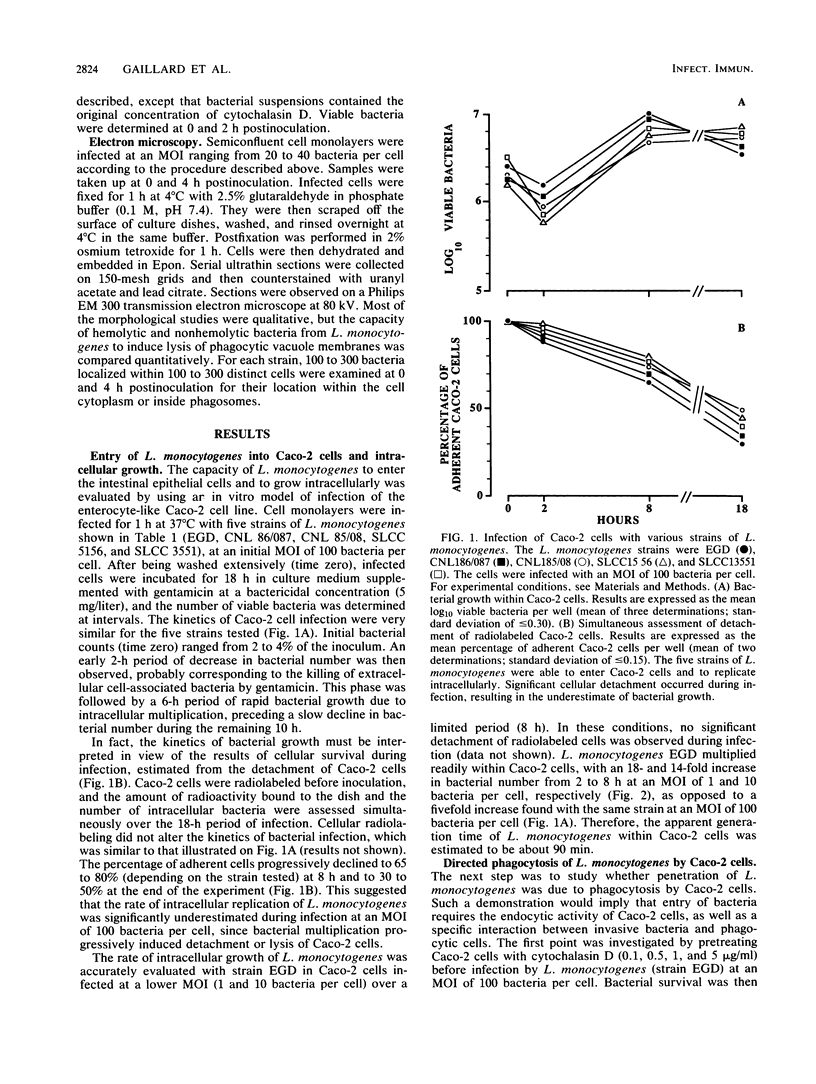
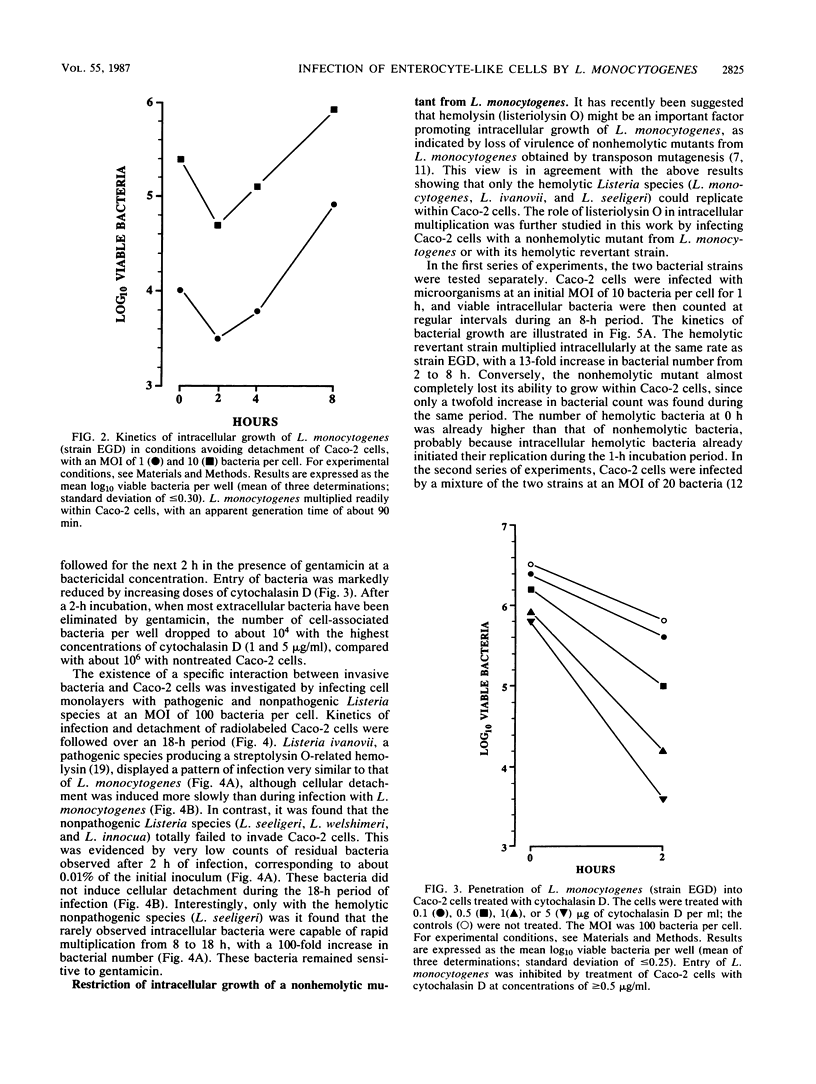
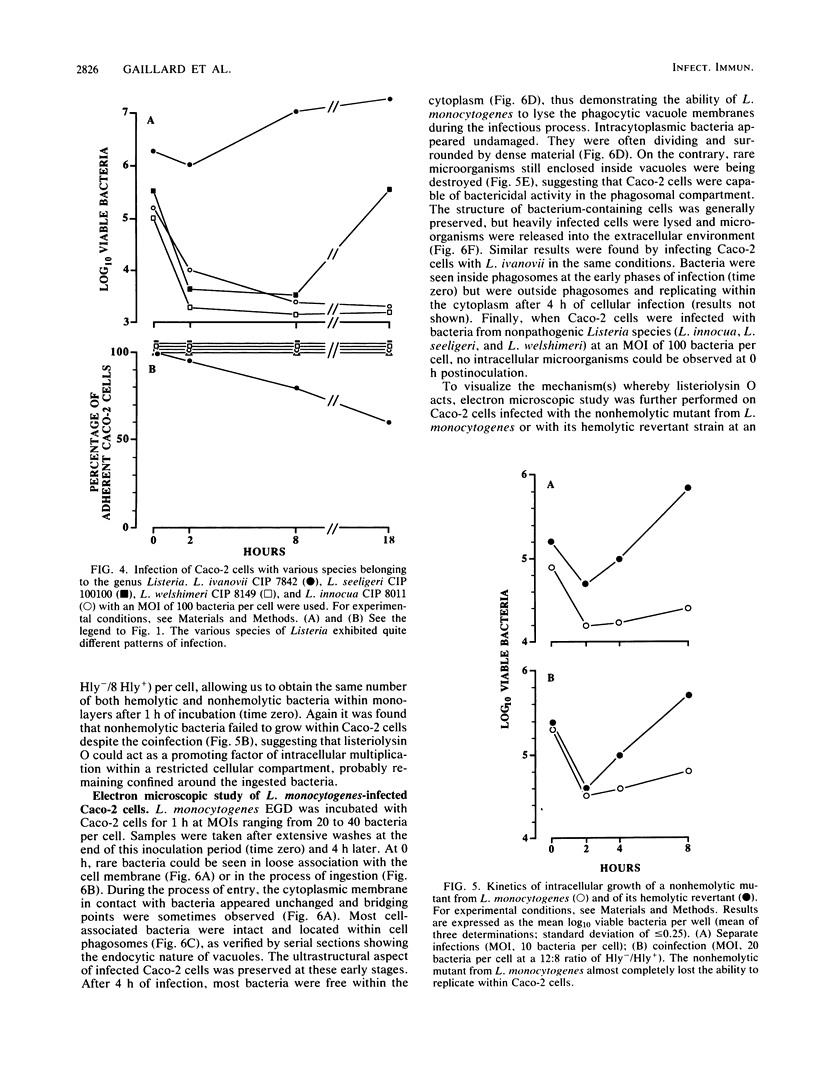
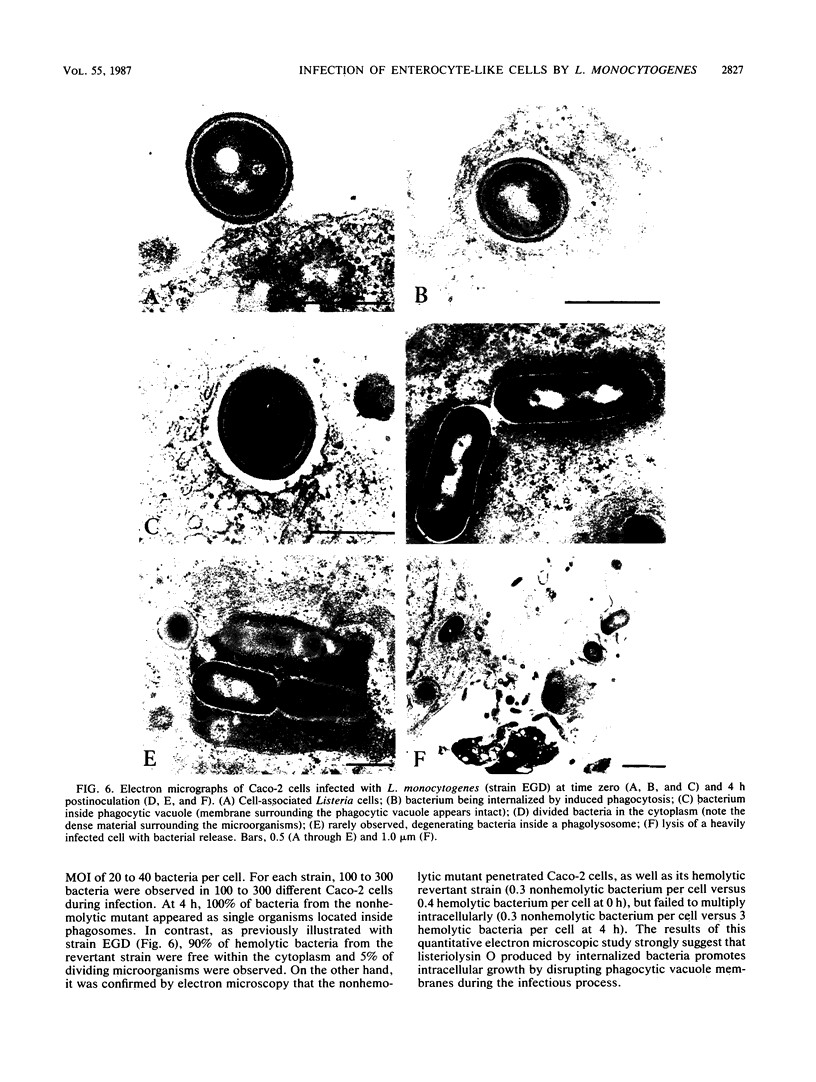
Penetration and replication of Listeria monocytogenes within intestinal epithelial cells were studied by infecting the human enterocyte-like cell line Caco-2. Entry was due to directed phagocytosis, as suggested by the inhibiting effect of cytochalasin D on bacterial entry and by electron microscopy showing bacteria inside membrane-limiting vacuoles at the early stage of infection. Only bacteria from pathogenic species (L. monocytogenes and Listeria ivanovii) were able to induce their own phagocytosis by Caco-2 cells, as opposed to Listeria seeligeri, Listeria welshimeri, and Listeria innocua. L. monocytogenes multiplied readily within Caco-2 cells, with an apparent generation time of about 90 min. Listeriolysin O was found to be a major factor promoting intracellular growth of L. monocytogenes. After being internalized at the same rate as that of its hemolytic revertant strain, a nonhemolytic mutant from L. monocytogenes failed to replicate significantly within Caco-2 cells. Electron microscopic study demonstrated that bacteria from the nonhemolytic mutant remained inside phagosomes during cellular infection, whereas hemolytic bacteria from L. monocytogenes were released free within the cytoplasm. This indicates that disruption of vacuole membranes by listeriolysin O-producing strains of L. monocytogenes might be a key mechanism allowing bacteria to escape from phagosomes and to multiply unrestricted within cell cytoplasm.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong B. A., Sword C. P. Electron microscopy of Listeria monocytogenes-infected mouse spleen. J Bacteriol. 1966 Mar;91(3):1346–1355. doi: 10.1128/jb.91.3.1346-1355.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. L., Killinger A. H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966 Jun;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon G. C., Sword C. P. Effects of Listeria monocytogenes Hemolysin on Phagocytic Cells and Lysosomes. Infect Immun. 1970 Apr;1(4):356–362. doi: 10.1128/iai.1.4.356-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., Warner S., Amell L. Listeria pneumonitis: influence of route of immunization on resistance to airborne infection. Infect Immun. 1979 Aug;25(2):672–679. doi: 10.1128/iai.25.2.672-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Carter P. B. Cell-mediated immunity to intestinal infection. Infect Immun. 1980 May;28(2):516–523. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSEBOLD J. W., INOUYE T. Pathogenesis of Listeria monocytogenes infections in natural host. I. Rabbit studies. J Infect Dis. 1954 Jul-Aug;95(1):52–66. doi: 10.1093/infdis/95.1.52. [DOI] [PubMed] [Google Scholar]
- OSEBOLD J. W., INOUYE T. Pathogenesis of Listeria monocytogenes infections in natural hosts. II. Sheep studies. J Infect Dis. 1954 Jul-Aug;95(1):67–78. doi: 10.1093/infdis/95.1.67. [DOI] [PubMed] [Google Scholar]
- Parrisius J., Bhakdi S., Roth M., Tranum-Jensen J., Goebel W., Seeliger H. P. Production of listeriolysin by beta-hemolytic strains of Listeria monocytogenes. Infect Immun. 1986 Jan;51(1):314–319. doi: 10.1128/iai.51.1.314-319.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Wands J., Trelstad R. L., Isselbacher K. J. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979 Feb;80(2):248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Ito S. Intracellular localization of Rickettsia tsutsugamushi in polymorphonuclear leukocytes. J Exp Med. 1979 Sep 19;150(3):703–708. doi: 10.1084/jem.150.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocourt J., Seeliger H. P. Distribution des espèces du genre Listeria. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 May;259(3):317–330. [PubMed] [Google Scholar]
- Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie. 1986 Sep;68(9):1035–1040. doi: 10.1016/s0300-9084(86)80177-8. [DOI] [PubMed] [Google Scholar]
- Rácz P., Kaiserling E., Tenner K., Wuthe H. H. Experimental Listeria cystitis. II. Further evidence of the epithelial phase in experimental Listeria infection. An electron microscopic study. Virchows Arch B Cell Pathol. 1973 May 15;13(1):24–37. [PubMed] [Google Scholar]
- Rácz P., Tenner K., Mérö E. Experimental Listeria enteritis. I. An electron microscopic study of the epithelial phase in experimental listeria infection. Lab Invest. 1972 Jun;26(6):694–700. [PubMed] [Google Scholar]
- Rácz P., Tenner K., Szivessy K. Electron microscopic studies in experimental keratoconjunctivitis listeriosa. I. Penetration of Listeria monocytogenes into corneal epithelial cells. Acta Microbiol Acad Sci Hung. 1970;17(3):221–236. [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Sword C. P. Mechanisms of pathogenesis in Listeria monocytogenes infection. I. Influence of iron. J Bacteriol. 1966 Sep;92(3):536–542. doi: 10.1128/jb.92.3.536-542.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. doi: 10.1128/iai.22.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Miller E. T. Phospholipase A and the interaction of Rickettsia prowazekii and mouse fibroblasts (L-929 cells). Infect Immun. 1982 Oct;38(1):109–113. doi: 10.1128/iai.38.1.109-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]