Abstract
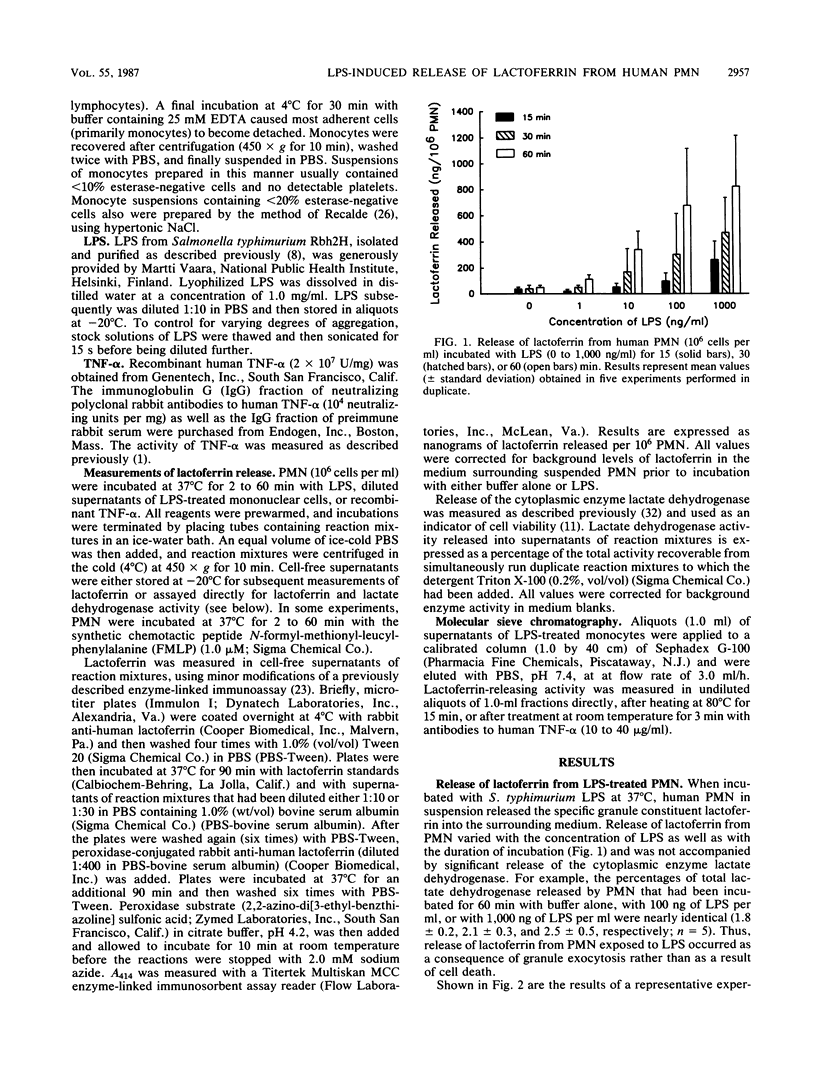
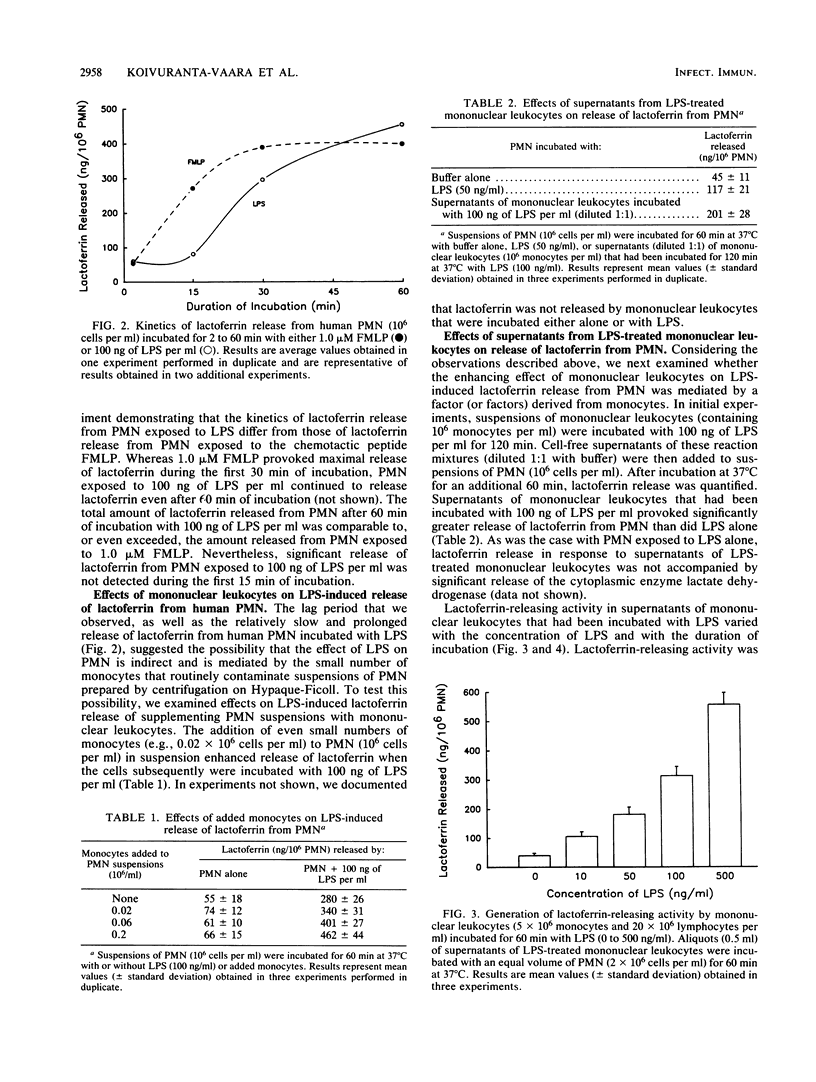
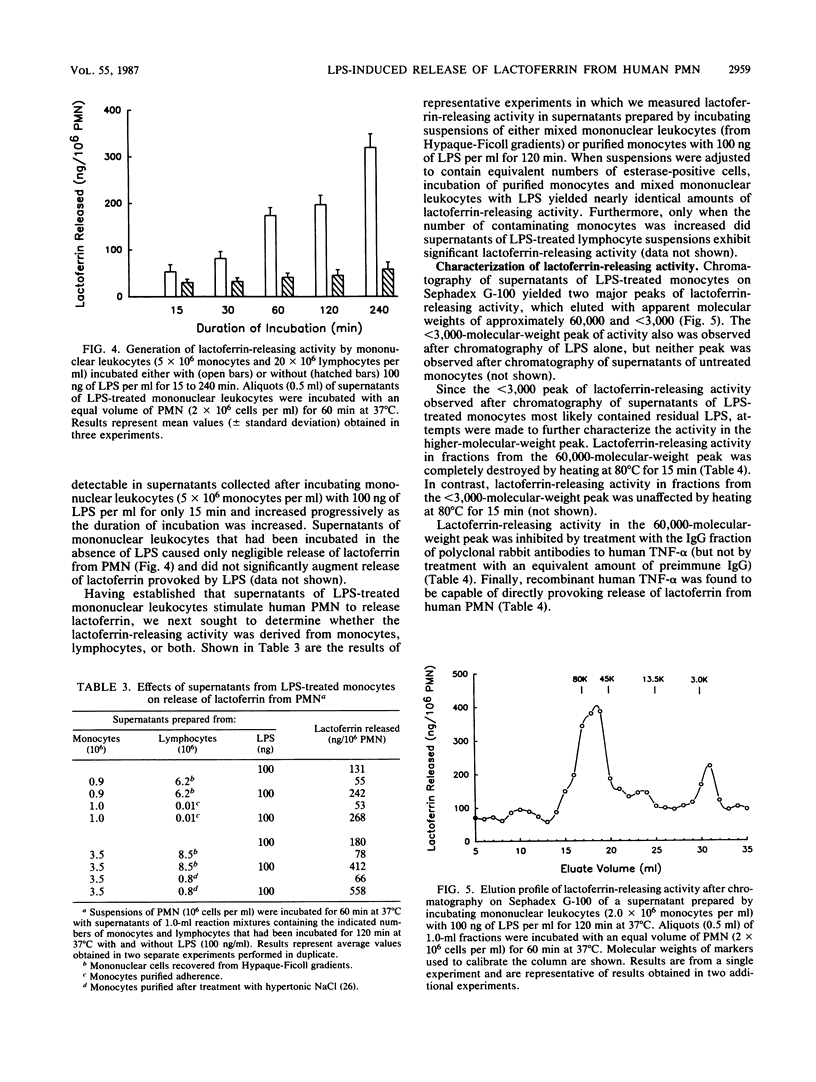
We have examined the role played by human peripheral blood monocytes in mediating responses of human polymorphonuclear leukocytes (PMN) to bacterial lipopolysaccharide (LPS) in vitro. When incubated with Salmonella typhimurium LPS at 37 degrees C, human PMN suspended in serum-free buffer released the specific granule constituent lactoferrin into the surrounding medium. Release of lactoferrin from PMN varied with the concentration of LPS (1 to 1,000 ng/ml) as well as with the duration of incubation (2 to 60 min) and was not accompanied by significant release of the cytoplasmic enzyme lactate dehydrogenase. LPS-induced release of lactoferrin from PMN was augmented significantly when cell suspensions were supplemented with additional monocytes and lymphocytes. Only monocytes, however, secreted significant amounts of lactoferrin-releasing activity (in a time- and concentration-dependent manner) when incubated separately with LPS. Lactoferrin-releasing activity was heat (80 degrees C for 15 min) labile, eluted after chromatography on Sephadex G-100 with an apparent molecular weight of approximately 60,000, and was inhibited by antibodies to tumor necrosis factor alpha. Thus, LPS-induced noncytotoxic release of lactoferrin from human PMN suspended in serum-free buffer is mediated, at least in part, by tumor necrosis factor alpha derived from contaminating monocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Arend W. P., Massoni R. J. Characteristics of bacterial lipopolysaccharide induction of interleukin 1 synthesis and secretion by human monocytes. Clin Exp Immunol. 1986 Jun;64(3):656–664. [PMC free article] [PubMed] [Google Scholar]
- Bannatyne R. M., Harnett N. M., Lee K. Y., Biggar W. D. Inhibition of the biologic effects of endotoxin on neutrophils by polymyxin B sulfate. J Infect Dis. 1977 Oct;136(4):469–474. doi: 10.1093/infdis/136.4.469. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cline M. J., Melmon K. L., Davis W. C., Williams H. E. Mechanism of endotoxin interaction with human leucocytes. Br J Haematol. 1968 Dec;15(6):539–547. doi: 10.1111/j.1365-2141.1968.tb01576.x. [DOI] [PubMed] [Google Scholar]
- Dahinden C., Fehr J. Granulocyte activation by endotoxin. II. Role of granulocyte adherence, aggregation, and effect of cytochalasin B, and comparison with formylated chemotactic peptide-induced stimulation. J Immunol. 1983 Feb;130(2):863–868. [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgilis K., Schaefer C., Dinarello C. A., Klempner M. S. Human recombinant interleukin 1 beta has no effect on intracellular calcium or on functional responses of human neutrophils. J Immunol. 1987 May 15;138(10):3403–3407. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Henricks P. A., van der Tol M. E., Thyssen R. M., van Asbeck B. S., Verhoef J. Escherichia coli lipopolysaccharides diminish and enhance cell function of human polymorphonuclear leukocytes. Infect Immun. 1983 Jul;41(1):294–301. doi: 10.1128/iai.41.1.294-301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S. T., Gennaro D. E., Manzi R. M. Neutrophils may directly synthesize both H2O2 and O2- since surface stimuli induce their release in stimulus-specific ratios. Inflammation. 1985 Dec;9(4):425–437. doi: 10.1007/BF00916342. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Allison A. C., Ward P., Kight N. Identification of human mononuclear leucocyte populations by esterase staining. Clin Exp Immunol. 1977 Nov;30(2):289–298. [PMC free article] [PubMed] [Google Scholar]
- Kapp A., Luger T. A., Maly F. E., Schöpf E. Granulocyte-activating mediators (GRAM): I. Generation by lipopolysaccharide-stimulated mononuclear cells. J Invest Dermatol. 1986 May;86(5):523–528. doi: 10.1111/1523-1747.ep12354953. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Klempner M. S., Dinarello C. A., Gallin J. I. Human leukocytic pyrogen induces release of specific granule contents from human neutrophils. J Clin Invest. 1978 May;61(5):1330–1336. doi: 10.1172/JCI109050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth R. S., Edgington T. S. Tumor necrosis factor production by human monocytes is a regulated event: induction of TNF-alpha-mediated cellular cytotoxicity by endotoxin. J Immunol. 1986 Oct 15;137(8):2585–2591. [PubMed] [Google Scholar]
- Larrick J. W., Graham D., Toy K., Lin L. S., Senyk G., Fendly B. M. Recombinant tumor necrosis factor causes activation of human granulocytes. Blood. 1987 Feb;69(2):640–644. [PubMed] [Google Scholar]
- Proctor R. A. Endotoxin in vitro interactions with human neutrophils: depression of chemiluminescence, oxygen consumption, superoxide production, and killing. Infect Immun. 1979 Sep;25(3):912–921. doi: 10.1128/iai.25.3.912-921.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalde H. R. A simple method of obtaining monocytes in suspension. J Immunol Methods. 1984 Apr 13;69(1):71–77. doi: 10.1016/0022-1759(84)90278-3. [DOI] [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Smith R. A., Baglioni C. The active form of tumor necrosis factor is a trimer. J Biol Chem. 1987 May 25;262(15):6951–6954. [PubMed] [Google Scholar]
- Smith R. J., Bowman B. J., Speziale S. C. Interleukin-1 stimulates granule exocytosis from human neutrophils. Int J Immunopharmacol. 1986;8(1):33–40. doi: 10.1016/0192-0561(86)90070-6. [DOI] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Jones D. P., Munkenbeck P., Morrison D. C. Serum-dependent and -independent effects of bacterial lipopolysaccharides on human neutrophil oxidative capacity in vitro. J Reticuloendothel Soc. 1982 Jan;31(1):43–57. [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]


