Abstract
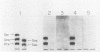
A fragment distinct from the heavy and light chains was obtained by treatment of Clostridium botulinum type B neurotoxin with chymotrypsin. Enzyme-linked immunosorbent assay and immunoblotting analysis with monoclonal antibodies showed that the fragment consisted of the light chain and part of the heavy chain (H-1 fragment) linked together by a disulfide bond. Monoclonal antibodies reacting to the heavy chain but not to the fragment were thought to recognize the epitopes on the remaining portion (H-2 fragment) of the heavy chain, being easily digested by chymotrypsin. Thus, the antigenic structure of type B neurotoxin resembles those of type A and E neurotoxins. The chymotrypsin-induced fragment bound to cerebroside and free fatty acids but not to gangliosides. The manner of binding of type B neurotoxin to gangliosides and free fatty acids was different from those of type A and E neurotoxins. Such differences in the reactivities to lipids may be related to the finding that each neurotoxin binds to a type-specific site on the neural membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandyopadhyay S., Clark A. W., DasGupta B. R., Sathyamoorthy V. Role of the heavy and light chains of botulinum neurotoxin in neuromuscular paralysis. J Biol Chem. 1987 Feb 25;262(6):2660–2663. [PubMed] [Google Scholar]
- Black J. D., Dolly J. O. Interaction of 125I-labeled botulinum neurotoxins with nerve terminals. I. Ultrastructural autoradiographic localization and quantitation of distinct membrane acceptors for types A and B on motor nerves. J Cell Biol. 1986 Aug;103(2):521–534. doi: 10.1083/jcb.103.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta B. R., Sugiyama H. A common subunit structure in Clostridium botulinum type A, B and E toxins. Biochem Biophys Res Commun. 1972 Jul 11;48(1):108–112. doi: 10.1016/0006-291x(72)90350-6. [DOI] [PubMed] [Google Scholar]
- Evans D. M., Williams R. S., Shone C. C., Hambleton P., Melling J., Dolly J. O. Botulinum neurotoxin type B. Its purification, radioiodination and interaction with rat-brain synaptosomal membranes. Eur J Biochem. 1986 Jan 15;154(2):409–416. doi: 10.1111/j.1432-1033.1986.tb09413.x. [DOI] [PubMed] [Google Scholar]
- Habermann E., Dreyer F. Clostridial neurotoxins: handling and action at the cellular and molecular level. Curr Top Microbiol Immunol. 1986;129:93–179. doi: 10.1007/978-3-642-71399-6_2. [DOI] [PubMed] [Google Scholar]
- Helting T. B., Zwisler O. Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J Biol Chem. 1977 Jan 10;252(1):187–193. [PubMed] [Google Scholar]
- Hoch D. H., Romero-Mira M., Ehrlich B. E., Finkelstein A., DasGupta B. R., Simpson L. L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata Y., Kozaki S., Sakaguchi G., Iwamori M., Nagai Y. Evidence for direct binding of Clostridium botulinum type E derivative toxin and its fragments to gangliosides and free fatty acids. Biochem Biophys Res Commun. 1986 Nov 14;140(3):1015–1019. doi: 10.1016/0006-291x(86)90736-9. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Iwamori M., Nagai Y. Interaction between Clostridium botulinum neurotoxin and gangliosides. Biochim Biophys Acta. 1980 Mar 20;628(3):328–335. doi: 10.1016/0304-4165(80)90382-7. [DOI] [PubMed] [Google Scholar]
- Kondo H., Shimizu T., Kubonoya M., Izumi N., Takahashi M., Sakaguchi G. Titration of botulinum toxins for lethal toxicity by intravenous injection into mice. Jpn J Med Sci Biol. 1984 Jun;37(3):131–135. doi: 10.7883/yoken1952.37.131. [DOI] [PubMed] [Google Scholar]
- Kozaki S. Interaction of botulinum type A, B and E derivative toxins with synaptosomes of rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jul;308(1):67–70. doi: 10.1007/BF00499721. [DOI] [PubMed] [Google Scholar]
- Kozaki S., Kamata Y., Nagai T., Ogasawara J., Sakaguchi G. The use of monoclonal antibodies to analyze the structure of Clostridium botulinum type E derivative toxin. Infect Immun. 1986 Jun;52(3):786–791. doi: 10.1128/iai.52.3.786-791.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki S., Miyazaki S., Sakaguchi G. Development of antitoxin with each of two complementary fragments of Clostridium botulinum type B derivative toxin. Infect Immun. 1977 Dec;18(3):761–766. doi: 10.1128/iai.18.3.761-766.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki S., Sakaguchi S., Sakaguchi G. Purification and some properties of progenitor toxins of Clostridium botulinum type B. Infect Immun. 1974 Oct;10(4):750–756. doi: 10.1128/iai.10.4.750-756.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi G. Clostridium botulinum toxins. Pharmacol Ther. 1982;19(2):165–194. doi: 10.1016/0163-7258(82)90061-4. [DOI] [PubMed] [Google Scholar]
- Sathyamoorthy V., DasGupta B. R. Separation, purification, partial characterization and comparison of the heavy and light chains of botulinum neurotoxin types A, B, and E. J Biol Chem. 1985 Sep 5;260(19):10461–10466. [PubMed] [Google Scholar]
- Schmidt J. J., Sathyamoorthy V., DasGupta B. R. Partial amino acid sequences of botulinum neurotoxins types B and E. Arch Biochem Biophys. 1985 May 1;238(2):544–548. doi: 10.1016/0003-9861(85)90198-5. [DOI] [PubMed] [Google Scholar]
- Shone C. C., Hambleton P., Melling J. Inactivation of Clostridium botulinum type A neurotoxin by trypsin and purification of two tryptic fragments. Proteolytic action near the COOH-terminus of the heavy subunit destroys toxin-binding activity. Eur J Biochem. 1985 Aug 15;151(1):75–82. doi: 10.1111/j.1432-1033.1985.tb09070.x. [DOI] [PubMed] [Google Scholar]
- Simpson L. L., Rapport M. M. Ganglioside inactivation of botulinum toxin. J Neurochem. 1971 Jul;18(7):1341–1343. doi: 10.1111/j.1471-4159.1971.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Simpson L. L. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981 Sep;33(3):155–188. [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Sugiyama H. Clostridium botulinum neurotoxin. Microbiol Rev. 1980 Sep;44(3):419–448. doi: 10.1128/mr.44.3.419-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamizawa K., Iwamori M., Kozaki S., Sakaguchi G., Tanaka R., Takayama H., Nagai Y. TLC immunostaining characterization of Clostridium botulinum type A neurotoxin binding to gangliosides and free fatty acids. FEBS Lett. 1986 Jun 9;201(2):229–232. doi: 10.1016/0014-5793(86)80614-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]