Abstract
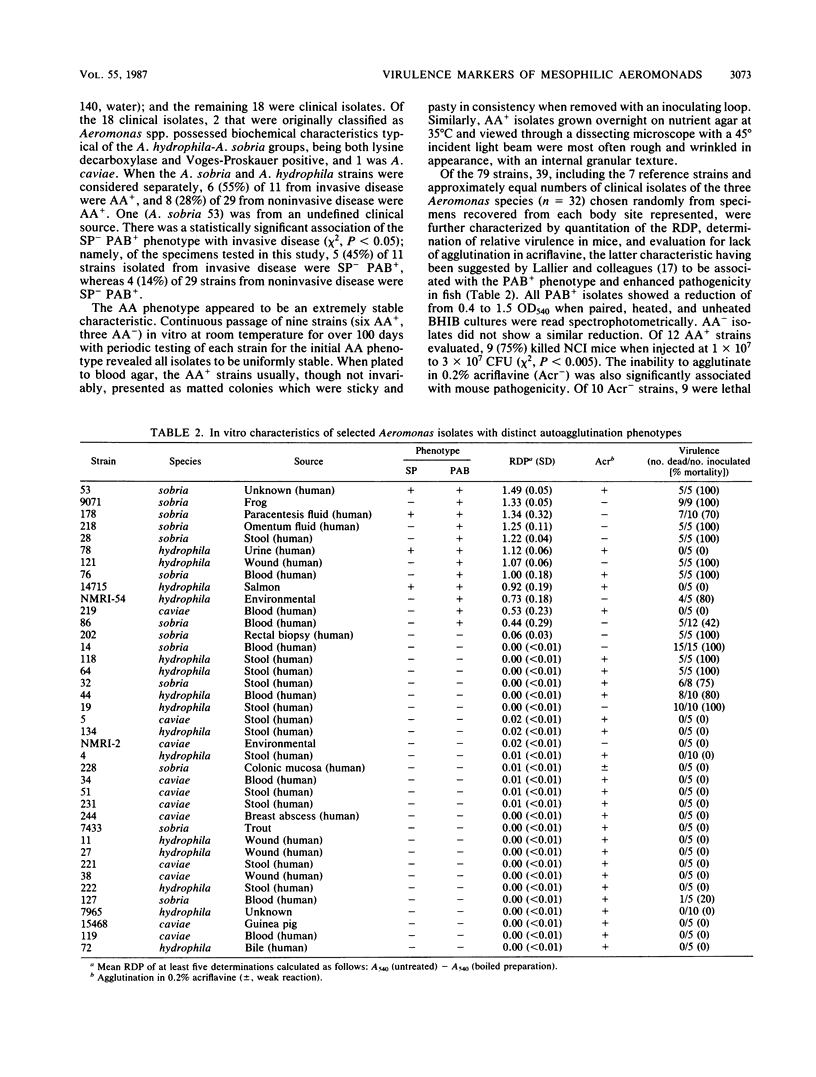
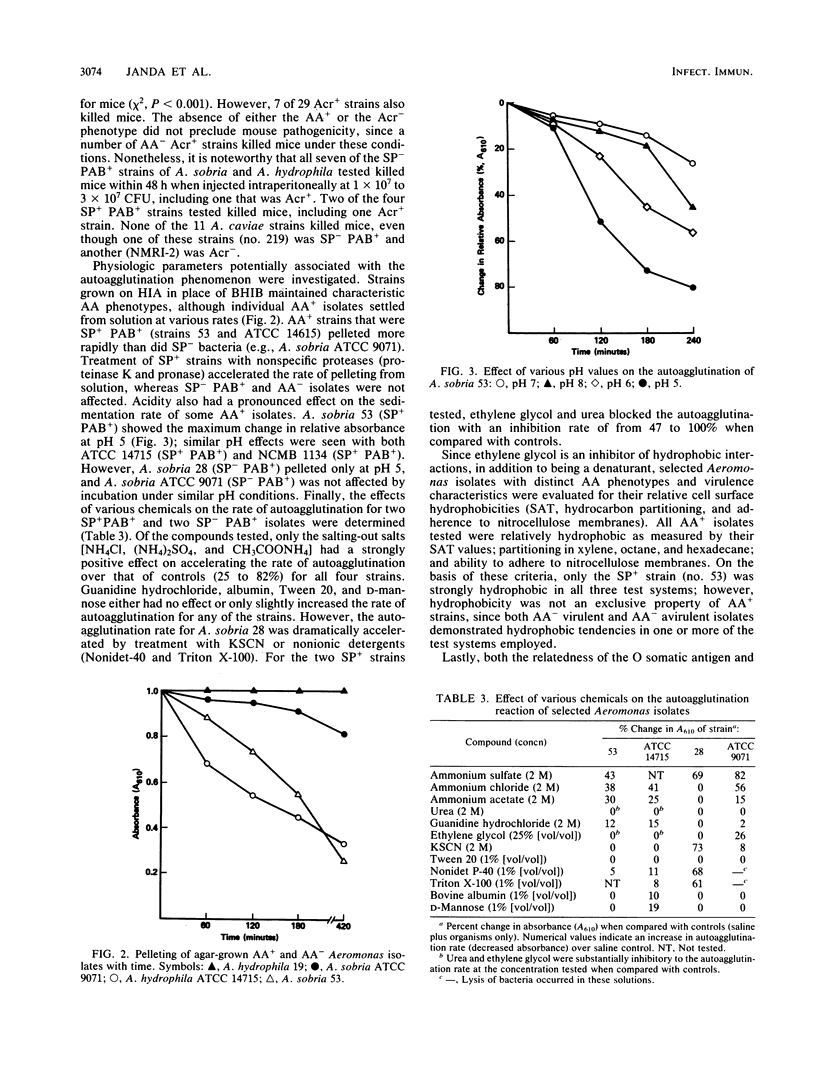
Autoagglutination (AA phenotype) of mesophilic aeromonads in broth was found to be a virulence-associated marker. There were two kinds of AA+ strains: those that spontaneously pelleted (SP+), and those that pelleted only after boiling (PAB+). Of 79 strains tested, 24 (30%) were AA+, and 18 of these were recovered from clinical specimens. Most of the AA+ strains (n = 21) were identified as either Aeromonas sobria or Aeromonas hydrophila. Of the well-documented clinical isolates of A. sobria and A. hydrophila available, 5 (46%) of 11 from invasive disease and 4 (14%) of 29 from noninvasive disease were SP- PAB+. The SP- PAB+ phenotype was significantly associated with invasive infections (e.g., bacteremia and peritonitis [chi 2, P less than 0.05]). All seven of the SP- PAB+ A. sobria and A. hydrophila strains tested killed mice within 48 h after intraperitoneal infection with 1 x 10(7) to 3 x 10(7) CFU, whereas only two of four SP+ PAB+ strains tested were lethal. All of the SP- PAB+ A. sobria and A. hydrophila isolates examined shared common O somatic antigens and possessed an external layer peripheral to the cell wall as determined by thin-section electron micrography. The LL1 strain of A. hydrophila used by Dooley et al. (J. S. G. Dooley, R. Lallier, and T. J. Trust, Vet. Immunol. Immunopathol. 12:339-344, 1986) to demonstrate an S membrane protein component in aeromonads virulent for fish also was SP- PAB+ and possessed the peripheral membrane, suggesting an association between these two components. Seven AA- and three SP+ strains tested lacked this layer; furthermore, 22 (71%) of 31 such isolates did not kill mice. The AA phenotype was a stable characteristic upon long-term passage of isolates in vitro. Study of SP+ and PAB+ aeromonads by surface charge and hydrophobicity analyses indicated that neither property correlated with either virulence or the presence of an external layer.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke V., Robinson J., Beaman J., Gracey M., Lesmana M., Rockhill R., Echeverria P., Janda J. M. Correlation of enterotoxicity with biotype in Aeromonas spp. J Clin Microbiol. 1983 Nov;18(5):1196–1200. doi: 10.1128/jcm.18.5.1196-1200.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily O. P., Joseph S. W., Coolbaugh J. C., Walker R. I., Merrell B. R., Rollins D. M., Seidler R. J., Colwell R. R., Lissner C. R. Association of Aeromonas sobria with human infection. J Clin Microbiol. 1981 Apr;13(4):769–777. doi: 10.1128/jcm.13.4.769-777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers L. M., Rees M. C., Turnbull A. C. Arachidonic acid metabolism by the non-pregnant human uterus. Prostaglandins Leukot Med. 1984 May;14(2):175–180. doi: 10.1016/0262-1746(84)90199-9. [DOI] [PubMed] [Google Scholar]
- Dooley J. S., Lallier R., Trust T. J. Surface antigens of virulent strains of Aeromonas hydrophila. Vet Immunol Immunopathol. 1986 Jun;12(1-4):339–344. doi: 10.1016/0165-2427(86)90138-8. [DOI] [PubMed] [Google Scholar]
- Evenberg D., Lugtenberg B. Cell surface of the fish pathogenic bacterium Aeromonas salmonicida. II. Purification and characterization of a major cell envelope protein related to autoagglutination, adhesion and virulence. Biochim Biophys Acta. 1982 Jan 22;684(2):249–254. doi: 10.1016/0005-2736(82)90013-x. [DOI] [PubMed] [Google Scholar]
- Evenberg D., Van Boxtel R., Lugtenberg B., Schurer F., Blommaert J., Bootsma R. Cell surface of the fish pathogenic bacterium Aeromonas salmonicida. I. Relationship between autoagglutination and the presence of a major cell envelope protein. Biochim Biophys Acta. 1982 Jan 22;684(2):241–248. doi: 10.1016/0005-2736(82)90012-8. [DOI] [PubMed] [Google Scholar]
- Freij B. J. Aeromonas: biology of the organism and diseases in children. Pediatr Infect Dis. 1984 Mar-Apr;3(2):164–175. [PubMed] [Google Scholar]
- Frye F. L. An unusual epizootic of anuran aeromoniasis. J Am Vet Med Assoc. 1985 Dec 1;187(11):1223–1224. [PubMed] [Google Scholar]
- Holmberg S. D., Farmer J. J., 3rd Aeromonas hydrophila and Plesiomonas shigelloides as causes of intestinal infections. Rev Infect Dis. 1984 Sep-Oct;6(5):633–639. doi: 10.1093/clinids/6.5.633. [DOI] [PubMed] [Google Scholar]
- Holmberg S. D., Schell W. L., Fanning G. R., Wachsmuth I. K., Hickman-Brenner F. W., Blake P. A., Brenner D. J., Farmer J. J., 3rd Aeromonas intestinal infections in the United States. Ann Intern Med. 1986 Nov;105(5):683–689. doi: 10.7326/0003-4819-105-5-683. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Bottone E. J., Reitano M. Aeromonas species in clinical microbiology: significance, epidemiology, and speciation. Diagn Microbiol Infect Dis. 1983 Sep;1(3):221–228. doi: 10.1016/0732-8893(83)90021-4. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Brenden R. Importance of Aeromonas sobria in Aeromonas bacteremia. J Infect Dis. 1987 Mar;155(3):589–591. doi: 10.1093/infdis/155.3.589. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Reitano M., Bottone E. J. Biotyping of Aeromonas isolates as a correlate to delineating a species-associated disease spectrum. J Clin Microbiol. 1984 Jan;19(1):44–47. doi: 10.1128/jcm.19.1.44-47.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica R. V., Zink D. L. Plasmid-associated cell surface charge and hydrophobicity of Yersinia enterocolitica. Infect Immun. 1984 May;44(2):540–543. doi: 10.1128/iai.44.2.540-543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc D., Mittal K. R., Olivier G., Lallier R. Serogrouping of motile Aeromonas species isolated from healthy and moribund fish. Appl Environ Microbiol. 1981 Jul;42(1):56–60. doi: 10.1128/aem.42.1.56-60.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Mittal K. R., Lalonde G., Leblanc D., Olivier G., Lallier R. Aeromonas hydrophila in rainbow trout: relation between virulence and surface characteristics. Can J Microbiol. 1980 Dec;26(12):1501–1503. doi: 10.1139/m80-248. [DOI] [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff M., Véron M. A taxonomic study of the Aeromonas hydrophila-Aeromonas punctata group. J Gen Microbiol. 1976 May;94(1):11–22. doi: 10.1099/00221287-94-1-11. [DOI] [PubMed] [Google Scholar]
- Shane S. M., Gifford D. H. Prevalence and pathogenicity of Aeromonas hydrophila. Avian Dis. 1985 Jul-Sep;29(3):681–689. [PubMed] [Google Scholar]
- Simpson L. M., White V. K., Zane S. F., Oliver J. D. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect Immun. 1987 Jan;55(1):269–272. doi: 10.1128/iai.55.1.269-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Chipman D. C. Clinical involvement of Aeromonas hydrophila. Can Med Assoc J. 1979 Apr 21;120(8):942–946. [PMC free article] [PubMed] [Google Scholar]
- Watson I. M., Robinson J. O., Burke V., Gracey M. Invasiveness of Aeromonas spp. in relation to biotype, virulence factors, and clinical features. J Clin Microbiol. 1985 Jul;22(1):48–51. doi: 10.1128/jcm.22.1.48-51.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]




