Abstract
(1) Objective
The myeloproliferative disorders (MPD), polycythemia vera (PV), essential thrombocytosis (ET) and primary myelofibrosis (PMF) differ phenotypically but share the same JAK2V617F mutation. We examined the relationship of the quantitative JAK2V617F allele burden to MPD disease phenotype among the three MPD classes and within PV.
(2) Methods
We measured the JAK2V617F allele percentage in genomic DNA from neutrophils, CD34+ cells, and cloned progenitors in 212 JAK2V617F –positive MPD patients and correlated the allele burdens to both disease class and disease features.
(3) Results
In ET and PV, the mean CD34+ cell JAK2V617F allele burdens were lower than the corresponding neutrophil allele burdens, but these were equivalent in PMF. JAK2WT progenitors were present in ET and PV when the CD34+ JAK2V617F allele burden was lower than the neutrophil allele burden, but not in PV and PMF subjects in whom the CD34+ cell and neutrophil allele burdens were similar. CD34+ cell JAK2V617F clonal dominance, defined as coherence between the CD34+ cell and neutrophil JAK2V617F allele burdens, was present in 24% of ET, 56% of PV and 93% of PMF patients, and was independent of the CD34+ cell JAK2V617F genotype. Clonally-dominant PV patients had significantly longer disease durations, higher white cell counts and larger spleens than nondominant PV patients.
(4) Conclusions
We conclude that the extent of JAK2V617F CD34+ cell clonal dominance is associated with disease phenotype within the MPD, and in PV, is associated with extramedullary disease, leukocytosis and disease duration.
Introduction
Polycythemia vera (PV), primary myelofibrosis (PMF) and essential thrombocytosis (ET) are clonal myeloproliferative disorders that share in common origin in a multipotent hematopoietic progenitor cell(1), cytogenetic abnormalities of chromosomes 1, 8, 9, 13 and 20(2), and growth factor-independent in vitro hematopoietic colony formation(3). Taken together, these shared phenotypic and genotypic features suggest that PV, PMF and ET might not be separate disorders but rather, different manifestations of the same disease. The recent discovery of the same JAK2 activating mutation (JAK2V617F) in all three disorders supported this contention(4–6).
However, PV, ET and PMF each have a unique epidemiology and natural history. Moreover, despite the same genetic lesion, the quantitative JAK2V617F neutrophil and platelet allele burdens are variable due to gene dosage in individual cells and the fact that many JAK2V617F -positive myeloproliferative disorder (MPD) patients retain JAK2 wild-type clones (JAK2WT)(7–10). Clonal dominance with suppression of normal hematopoietic progenitors is a feature of the MPD(7) and the extent of this could be responsible for the different clinical manifestations of PV, ET and PMF. To examine this possibility we analyzed the relationship of JAK2V617F allele burden measured in peripheral blood CD34+ cells, cloned CD34+ colonies and neutrophils to clinical phenotype in PV, PMF and ET. We observed significantly lower neutrophil and CD34+ cell JAK2V617F allele burdens in ET compared to either PV or PMF. Importantly, we also observed that in ET patients and many PV patients, the CD34+ cell JAK2V617F allele burdens were lower than the corresponding neutrophil allele burdens, and that this was due to the presence of JAK2WT CD34+ progenitor cells. In contrast, in PMF, CD34+ cell and neutrophil JAK2V617F allele burdens were similar, and clonal dominance of JAK2V617F –positive CD34+ cells was the rule.
The presence of wild-type CD34+ progenitor cells was associated with less extramedullary disease regardless of disease class, and in PV, was associated with shorter disease duration. Importantly, in PV the CD34+ cell JAK2V617F allele burden correlated better with disease duration, leukocytosis and extramedullary disease than did the neutrophil JAK2V617F allele burden. These data support a model of clonal expansion of JAK2V617F-positive CD34+ progenitor cells that distinguishes ET and PV from PMF. Within PV, clonal dominance at the CD34+ progenitor cell level is time-dependent and associated with extramedullary disease.
Methods
Subjects
The study protocol was approved by our Joint Committee on Clinical Investigation and written informed consent was obtained from each patient. The diagnosis of PV and ET were based on the Polycythemia Vera Study Group criteria and included an elevated red cell mass obtained at the time of diagnosis or follow-up as indicated (11). The diagnosis of PMF(12) was based on the Italian Consensus Conference (13). The patients recruited to this study were evaluated in our Center for the Chronic Myeloproliferative Disorders and all blood samples were obtained between 5/2005 and 1/2008. Clinical parameters including cell counts, family history, chemotherapy and spleen size were extracted from the medical record generated at the time blood was collected for analysis. Splenomegaly was considered present if the spleen was palpable by physical exam and was reported as centimeters (cm) below the costal margin with a maximum of 14 cm; patients who had undergone splenectomy were also given a score of 14 cm.
Cell Processing and CD34+ Cell Culture
Neutrophil isolation and DNA preparation were performed as described previously(14). Peripheral blood CD34+ cells were isolated from Ficoll buffy coats using immunomagnetic beads (Miltenyi, Auburn, CA). CD34+ cells were analyzed for the expression of CD34, CD38, CD117 and CD41 using commercially available labeled antibodies. Fluorescence of at least 10,000 cells was measured on a FACS Caliber and analyzed with Cellquest and Paint-a-gate software (BD Biosciences, San Jose, CA). Similar to other published reports of the CD34+ cell phenotype in the MPD(15), the peripheral blood CD34+ cells were greater than 95% positive both for CD34 and CD38, were dimly positive for CD117, were CD41 negative, and were primarily in Go/G1 of the cell cycle. Purified peripheral blood CD34+ cells were cultured using Methocult semisolid media with growth factors (Stem Cell Technologies, Vancouver, Canada) according to the manufacturer’s recommendations and incubated in a humidified environment at 37°C for 12 days. BFU-E and CFU-GM colonies, identified by visual inspection, were plucked for isolation of genomic DNA.
JAK2V617F Determination
Quantitative JAK2V617F allele percentage was performed as previously described using an allele-specific, quantitative real-time PCR assay sensitive to 10% of either the wild-type or mutant JAK2 allele(14).
Statistical Analysis
We used the t-test for continuous variables and the chi-square test for categorical variables as appropriate. P-values less than or equal to 0.05 using a two-tailed test were considered significant. Statistical calculations were performed using SigmaStat (SSI, San Jose, CA).
Results
Neutrophil JAK2V617F allele burden associates with both disease class and disease manifestations
Table 1 shows the epidemiologic features of the 138 JAK2V617F -positive PV, 45 ET and 29 PMF patients. Marked splenomegaly, defined as greater than 8 cm below the costal margin by physical exam, was present in 17% of PV patients, and 7% required splenectomy. Of the 30% of patients on chemotherapy at the time of the study, 82% received hydroxyurea, 11% interferon and 6% anagrelide. The median disease duration at the time of analysis for the entire cohort was 7 years; 22% of the patients were studied at one year or less from their diagnosis. As observed previously, neutrophil JAK2V617F allele burdens were significantly lower in ET compared to PV or PMF (14;16–18); women had lower neutrophil JAK2V617F allele burdens compared to men in ET and PV, although this was only significant in PV (p=0.01) , while women had significantly higher neutrophil allele burdens compared to men in PMF (p=.02) (Table 1).
Table 1.
Clinical and epidemiologic features of the JAK2V617F -positive MPD cohort.
| ET | PV | PMF | |
|---|---|---|---|
| N | 45 | 138 | 29 |
| % female | 66 | 64 | 39 |
| % antecedent MPD↓ | NA | 20 | 27 |
| Median disease duration, years (range) | 4 (0–21)§ | 8 (0–28) | 7 (0–20) |
| % with family MPD history | 22 | 6 | 6 |
| % with palpable splenomegaly | 6 | 44 | 100 |
| % receiving chemotherapy | 27 | 31 | 21 |
| Mean age at diagnosis | |||
| Females (range) | 53 (27–84) | 51 (15–88)* | 57 (27–70) |
| Males (range) | 52 (21–78) | 57 (30–74) | 58 (53–78) |
| Mean neutrophil JAK2V617F % | |||
| Total | 38# | 68 | 70 |
| Females (range) | 36 (13–61) | 68 (29–100)† | 82 (45–100)γ |
| Males (range) | 41 (17–63) | 74 (32–100) | 62 (43–100) |
20% of both PV and PMF patients had a prior history of ET, while 7% of the PMF patients had a prior history of PV.
Median disease duration ET vs PV, p= 0.006.
Age at PV diagnosis, female versus male, p = .04
Allele burden PV vs ET, p = <.001
female vs male PMF, p=.02
We tested the association of the quantitative neutrophil JAK2V617F allele burden to disease features including disease duration at the time of cell collection, platelet count, reticulocyte count spleen size and white cell count in 33 ET, 94 PV and 23 PMF patients who were not receiving chemotherapy. In ET and PMF, the neutrophil allele burden significantly correlated only with the white cell count (R=.44, p=.01 in ET and R=.50, p=.02 in PMF; Supplemental data Figure S1). In PV, the neutrophil JAK2V617F allele burden inversely correlated with platelet count (R=.31, p=.03), and directly correlated with the absolute reticulocyte count (R=.57, p=<.001), white cell count (R=.64, p=<.001) and spleen size (r=.42, p=.002; Supplemental data Figure S1).
CD34 cell JAK2V617F allele burden associates with both disease class and disease manifestations
JAK2V617F -positive cells could predominate in the peripheral blood due to either an advantage in terminal differentiation of the JAK2V617F -positive committed progenitor cells over residual normal committed progenitors(8;19;20), or due to the clonal expansion of JAK2V617F -positive progenitor cells(21) at the progenitor level. Measuring the burden of JAK2V617F at the progenitor level should distinguish between these possibilities and might prove to be more informative with regard to association with disease endpoints. CD34+ cells isolated from peripheral blood of MPD patients provide access to such a progenitor population, behave biologically similarly to marrow-derived MPD(22) progenitors and can be assessed for all three phenotypes, PV, PMF and ET, whereas marrow derived CD34+ cells are generally not available in PMF patients and many PV patients. We, therefore, assessed the relationship of the peripheral blood CD34+ JAK2V617F allele burden to disease phenotype in 96 MPD patients. As shown in Figure 1, CD34+ JAK2V617F cell allele burdens were significantly lower in ET compared to PV or PMF. CD34+ cell JAK2V617F allele percentages were significantly lower compared to neutrophil allele percentages in ET and PV, while there was no significant difference between neutrophil and CD34+ cell JAK2V617F allele percentages in PMF.
Figure 1.

Quantitative JAK2V617F allele burden in neutrophils and CD34+ cells obtained from the same blood sample in 17 ET, 64 PV and 15 PMF patients. Medians are indicated by bars, coincident points are overlapping.
Within PV, similar to the findings in neutrophils, the CD34+ cell JAK2V617F allele burden inversely correlated with platelet count (R=.34, p=.02), and directly correlated with the absolute reticulocyte count (R=.57, p=<.001), white cell count (R=.70, p=<.001) and splenomegaly (R=.37, p=.008) (Supplemental Figure S2). In contrast to the findings in neutrophils, however, the CD34+ cell JAK2V617F allele burden significantly increased over time (R=.45, p=<.001) (Supplemental Figure S2).
CD34+ cell progeny recapitulated the CD34+ JAK2V617F allele burden
In the majority of the ET and approximately half of the PV patients, the CD34+ cell JAK2V617F allele burdens were less than 50%, suggesting the presence of JAK2WT progenitor cells (Figure 1). To further define this observation, we cultured CD34+ cells from 6 ET, 13 PV and 6 PMF patients and determined the individual JAK2V617F genotype in twenty to forty burst-forming-units-erythroid (BFU-e) from each patient.
The results for the 6 ET, 13 PV and 6 PMF patients studied in this fashion are presented in Figure 2, sorted by disease class and decreasing percentage of JAK2WT clones. JAK2WT clones were present in more than 50% of the colonies tested in the majority of ET and many PV patients, but this was not observed in the PMF patients. JAK2V617F homozygous BFU-e clones were present primarily in PV and PMF cases (only one clone in one ET patient was homozygous) and while homozygous clones were mainly restricted to PV and PMF cases, not all PV and PMF cases were characterized by the presence of homozygous clones; a considerable proportion of PV and PMF patients had predominantly heterozygous colonies (3/13 PV, 2/6 PMF), a percentage that is identical to a recently published report (8). The presence of all three possible clones, JAK2WT, JAK2V617F heterozygous and JAK2V617F homozygous, was a feature of the majority of PV patients (8/13 patients), while this was not observed in either the ET or PMF patients. We observed that in PV, with the exception of case 7, the presence of greater than 50% JAK2WT clones was associated with the absence of splenomegaly and disease durations of less than ten years.
Figure 2.

Relationship of BFUe JAK2V617F genotypes to MPD subtype, disease duration, sex, splenomegaly, and measured CD34+ cell and neutrophil JAK2V617F allele burdens in 25 patients. On average, 27 colonies were plucked from each cloning experiment (range 20–40). Clear bars indicate a JAK2WT genotype, striped a heterozygous JAK2V617F genotype and black a homozygous JAK2V617F genotype. Asterisks above case number (top) indicate antecedent history of ET in all cases with the exception of 25, where there was an antecedent history of PV.
In the majority of ET and many of the PV patients, the neutrophil JAK2V617F allele burden measured from the same blood sample from which the CD34+ cells were isolated was much higher than the calculated CD34+ cell JAK2V617F allele burden derived from JAK2 genotypes of the cloned BFU-e, or from the measured CD34+ cell allele burden from an aliquot of CD34+ cells from the same cell isolation used for the BFU-e cloning experiment (Figure 2), such that the neutrophil allele burden overestimated the size of the JAK2V617F -positive clone at the CD34+ cell level. In contrast, in PMF, the neutrophil JAK2V617F allele burden better reflected the disease clone genotype because the number of residual JAK2WT colonies was very low (Figure 2). Indeed, the correlations between the neutrophil and CD34 cell JAK2V617F allele burdens varied between the MPD disease classes, with correlation coefficients of 0.96 in PMF 0.80 in PV and 0.67 in ET (Figure 3). The significance of differences between the three regressions was evaluated by an analysis of covariance, which found a significant difference between the regressions of PMF versus PV (p=<0.0006) and PMF versus ET (p=.000001), whereas there was no significant difference between that of PV versus ET.
Figure 3.
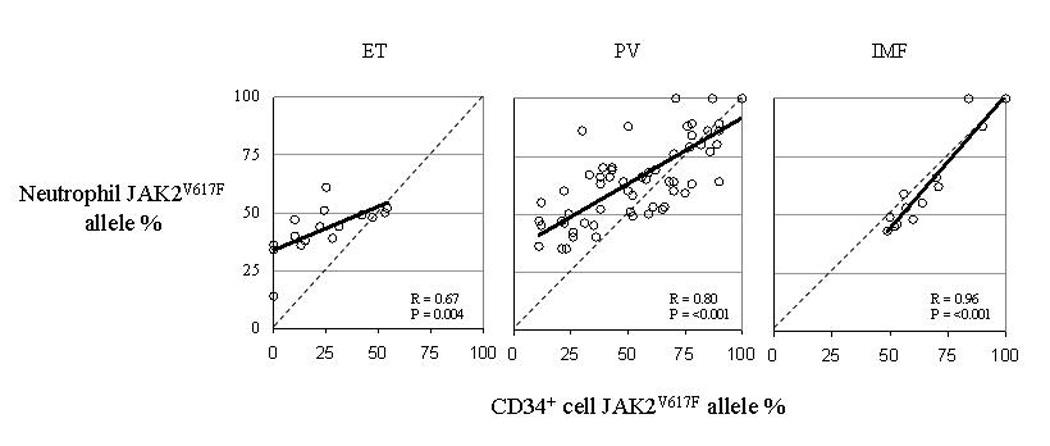
Relationship of Neutrophil JAK2V617F allele burden to CD34 cell JAK2V617F allele burden in 17 ET, 64 PV and 15 PMF patients. Dotted line indicates the regression line for an R=1.0 for reference.
JAK2V617F Clonal Dominance at the CD34+ Cell Level Associates with disease class and disease features within PV
In some ET, PV and PMF subjects only JAK2V617F -positive clones were present, and we defined these subjects to be JAK2V617F clonally dominant at the progenitor level (Figure 2). We observed that in these particular subjects (subjects 5,6,16–19,22–25) the absolute numerical difference between the measured neutrophil and CD34+ cell JAK2V617F allele burdens was 10 or less (Figure 2). We therefore arbitrarily defined an absolute difference between the quantitative neutrophil and CD34+ JAK2V617F allele burdens of 10 or less as an indicator of “clonal dominance” at the progenitor level, and tested the associations of clonal dominance between disease classes and within PV disease features in 17 ET, 64 PV and 15 PMF patients. The prevalence of clonal dominance was significantly different between the three disease classes, present in 4/17 (24%) ET, 36/64 (56%) PV and 14/15 (93%) PMF patients (p<0.05). PV patients who were clonally nondominant at the progenitor level had significantly shorter disease durations, lower white cell counts, and smaller spleens compared to PV patients who were clonally dominant at the progenitor level (Table 2). Importantly, the neutrophil allele burdens in the clonally dominant group were not significantly different than the nondominant group (mean 68.1 vs 62.5%; p =0.29) and therefore, could not reliably distinguish these two groups (Table 2).
Table 2.
Comparison of clinical features of clonally dominant versus nondominant PV patients in 50 patients not receiving chemotherapy.
| Clonally dominant PV | Clonally nondominant PV | P value | |
|---|---|---|---|
| Number | 26 | 24 | |
| Disease duration (years) | 8.1 (0–25) | 3.0 (0–10) | 0.007 |
| Platelet count (K/cu mm) | 526 (38–1239) | 663 (291–1269) | 0.12 |
| Reticulocyte count (K/cu mm) | 102.3 (45.5–232.2) | 94.6 (62.2–138.2) | 0.43 |
| Spleen size (centimeters below the costal margin) | 4.3 (0–14) | 1.3 (0–14) | 0.05 |
| White cell count (K/cu mm) | 20.4 (4.5–47.7) | 13.4 (5.8–37.2) | 0.01 |
| Neutrophil allele burden (%) | 68.1 (40–100) | 62.5 (35–100) | 0.29 |
| CD34 cell allele burden (%) | 70.5 (35–100) | 36.3 (11–87) | <.001 |
All values are expressed as means and ranges (parentheses).
Discussion
The World Health Organization (WHO) currently classifies PV, PMF and ET together with chronic myelogenous leukemia, chronic eosinophilic leukemia, chronic neutrophilic leukemia, the hypereosinophilic syndrome and unclassifiable chronic myeloproliferative disease under the rubric of the chronic myeloproliferative disorders(23). However, PV, PMF and ET have more in common phenotypically with each other than with these other disorders and recently this association was confirmed by the discovery of the JAK2V617F mutation, the expression of which is largely confined to PV, PMF and ET(24;25).
If one assumes that JAK2V617F is central to MPD pathogenesis, the observation that different disease classes associate with the identical mutation requires explanation. The issues that may be important to consider with respect to this are the gene dosage within the disease clone, and the extent of clonal dominance of that clone over the normal polyclonal hematopoiesis. In contrast to CML, in which the BCR/ABL-positive clone is uniformly dominant at the neutrophil level, in ET, PV and PMF, clonal dominance at the circulating blood cell level is not always observed(26–28) . To examine these issues, we measured the percentage of JAK2V617F alleles in PV, PMF and ET neutrophils, CD34+cells, and cultured erythroid colonies using a quantitative, allele-specific assay. Our study population consisted of a large, clinically well-defined group of PV, PMF and ET patients followed for up to 30 years.
Our observation in PV and ET that the neutrophil JAK2V617F allele burden was often much higher than the CD34+ cell allele burden indicated that JAK2V617F is advantageous to the terminal stages of hematopoietic differentiation, a finding recently reported by others(8). Thus, in PV and ET, the neutrophil JAK2V617F allele burden can over-estimate the burden at the progenitor cell level, which can be variable in both ET and PV (Figure 1–Figure 3). Moreover, JAK2V617F has been identified only in platelets in some ET patients, and at higher levels in some of these patients compared to their neutrophils, again suggesting that JAK2V617F may also be differentially advantageous to specific myeloid lineages at their terminal maturation stage(29–31).
In PV, an analysis of the CD34+ cell JAK2V617F allele burden revealed a stronger correlation to disease endpoints such as splenomegaly, white cell count and platelet count than the neutrophil JAK2V617F allele burden, and also revealed that the CD34+ cell JAK2V617F allele burden increased over time in PV, as was previously demonstrated using G-6PD isoenzyme analysis(7). The fact that CD34+ JAK2V617F allele burden more strongly correlated with some disease features than the neutrophil JAK2V617F allele burden in PV indicates that the expansion or dominance of JAK2V617F-positive over JAK2V617F -negative cells at the CD34+ cell level is an important biologic factor in the evolution of this disorders, a finding also observed in murine models of JAK2V617F -positive myeloproliferative disease(32). In contrast, in PMF, the JAK2V617F -bearing clone was dominant at the progenitor level independent of disease duration. Thus, the JAK2V617F -positive clone behaves differently biologically in de novo PMF compared to ET or PV, since it exhibits clonal dominance and expansion at disease onset, a feature reflected in the increase in circulating CD34+ cells in the early stages of PMF(15).
Our data also speak to the assessment of JAK2V617F-targeted therapy in the MPD. Studies in JAK2 knockout mice have indicated that JAK2 is not obligatory for primitive stem cell proliferation and lineage-specific commitment but rather, only for the survival of committed erythroid, myeloid and megakaryocytic cells(33). Thus, JAK2V617F enhanced the terminal amplification and development of committed cells in the erythroid lineage (3;34), granulocyte lineage(35) and platelets(30;31), and made committed myeloid progenitor cells hypersensitive to hydroxyurea(36). However, neither hydroxyurea(37;38) nor other forms of chemotherapy(39–41) prevent MPD progression. Therefore, therapies directed against the JAK2V617F -bearing clones will have to be active at the primitive progenitor level in order to do more than suppress disease activity, and analysis of therapeutic efficacy will require assessment for residual disease at the primitive progenitor cell level.
We conclude that JAK2V617F clones behave biologically differently at the stem cell level in ET, PV and PMF, and that the degree of expansion or domination at the CD34+ progenitor cell level is an important modifier of the MPD disease phenotype. However, given that these diseases are indolent, often requiring decades to fully declare their phenotype in an individual patient, genetic, gender and environmental factors that influence the behavior of the disease clone at the stem cell level will also be important to identify as they may provide clinically relevant diagnostic and prognostic information(42–44).
Supplementary Material
S1. Association of the neutrophil JAK2V617F allele burden to disease features in 33 ET (left panels), 94 PV (center panels), and 23 PMF (right panels) patients not receiving chemotherapy. Correlations which reached statistical significance are indicated by the presence of the linear regression line, and the R and p values.
S2. Associations of the neutrophil and CD34+ cell JAK2V617F allele burdens to disease features in 50 PV patients not receiving chemotherapy. Correlations which reached statistical significance are indicated by the presence of the linear regression line, and the R and p values.
Acknowledgments
This work was supported by grants from the Myeloproliferative Disorders Foundation, the National Institutes of Health (RO1- HL082995), the Department of Defense (MPO 48019) and the National Cancer Institute (PO1-CA108671).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Adamson JW, Fialkow PJ, Murphy S, Prchal JF, Steinmann L. Polycythemia vera: stem-cell and probable clonal origin of the disease. N Engl J Med. 1976 October 21;295(17):913–916. doi: 10.1056/NEJM197610212951702. [DOI] [PubMed] [Google Scholar]
- 2.Najfeld V, Montella L, Scalise A, Fruchtman S. Exploring polycythaemia vera with fluorescence in situ hybridization: additional cryptic 9p is the most frequent abnormality detected. Br J Haematol. 2002 November;119(2):558–566. doi: 10.1046/j.1365-2141.2002.03763.x. [DOI] [PubMed] [Google Scholar]
- 3.Prchal JF, Axelrad AA. Letter: Bone-marrow responses in polycythemia vera. N Engl J Med. 1974 June 13;290(24):1382. doi: 10.1056/nejm197406132902419. [DOI] [PubMed] [Google Scholar]
- 4.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005 March 19;365(9464):1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 5.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J, Koo S, Lee JC, Gabriel S, Mercher T, D'Andrea A, Frohling S, Dohner K, Marynen P, Vandenberghe P, Mesa RA, Tefferi A, Griffin JD, Eck MJ, Sellers WR, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005 April;7(4):387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 6.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, naceur-Griscelli A, Villeval JL, Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005 April 28;434(7037):1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 7.Adamson JW, Singer JW, Catalano P, Murphy S, Lin N, Steinmann L, Ernst C, Fialkow PJ. Polycythemia vera. Further in vitro studies of hematopoietic regulation. J Clin Invest. 1980 December;66(6):1363–1368. doi: 10.1172/JCI109989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupont S, Masse A, James C, Teyssandier I, Lecluse Y, Larbret F, Ugo V, Saulnier P, Koscielny S, Le Couedic JP, Casadevall N, Vainchenker W, Delhommeau F. The JAK2 V617F mutation triggers erythropoietin hypersensitivity and terminal erythroid amplification in primary cells from patients with polycythemia vera. Blood. 2007 March 27; doi: 10.1182/blood-2006-10-054940. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto G, Nannya Y, Kato M, Sanada M, Levine RL, Kawamata N, Hangaishi A, Kurokawa M, Chiba S, Gilliland DG, Koeffler HP, Ogawa S. Highly sensitive method for genomewide detection of allelic composition in nonpaired, primary tumor specimens by use of affymetrix single-nucleotidepolymorphism genotyping microarrays. Am J Hum Genet. 2007 July;81(1):114–126. doi: 10.1086/518809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott LM, Scott MA, Campbell PJ, Green AR. Progenitors homozygous for the V617F mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood. 2006 October 1;108(7):2435–2437. doi: 10.1182/blood-2006-04-018259. [DOI] [PubMed] [Google Scholar]
- 11.Berk PD, Goldberg JD, Donovan PB, Fruchtman SM, Berlin NI, Wasserman LR. Therapeutic recommendations in polycythemia vera based on Polycythemia Vera Study Group protocols. Semin Hematol. 1986 April;23(2):132–143. [PubMed] [Google Scholar]
- 12.Mesa RA, Verstovsek S, Cervantes F, Barosi G, Reilly JT, Dupriez B, Levine R, Le Bousse-Kerdiles MC, Wadleigh M, Campbell PJ, Silver RT, Vannucchi AM, Deeg HJ, Gisslinger H, Thomas D, Odenike O, Solberg LA, Gotlib J, Hexner E, Nimer SD, Kantarjian H, Orazi A, Vardiman JW, Thiele J, Tefferi A. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMFBP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT) Leuk Res. 2007 June;31(6):737–740. doi: 10.1016/j.leukres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Barosi G. Myelofibrosis with myeloid metaplasia: diagnostic definition and prognostic classification for clinical studies and treatment guidelines. J Clin Oncol. 1999 September;17(9):2954–2970. doi: 10.1200/JCO.1999.17.9.2954. [DOI] [PubMed] [Google Scholar]
- 14.Moliterno AR, Williams DM, Rogers O, Spivak JL. Molecular mimicry in the chronic myeloproliferative disorders: reciprocity between quantitative JAK2 V617F and Mpl expression. Blood. 2006 December 1;108(12):3913–3915. doi: 10.1182/blood-2006-03-008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barosi G, Viarengo G, Pecci A, Rosti V, Piaggio G, Marchetti M, Frassoni F. Diagnostic and clinical relevance of the number of circulating CD34(+) cells in myelofibrosis with myeloid metaplasia. Blood. 2001 December 1;98(12):3249–3255. doi: 10.1182/blood.v98.12.3249. [DOI] [PubMed] [Google Scholar]
- 16.Vannucchi AM, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, Marchioli R, Marfisi RM, Finazzi G, Guerini V, Fabris F, Randi ML, De S, V, Caberlon S, Tafuri A, Ruggeri M, Specchia G, Liso V, Rossi E, Pogliani E, Gugliotta L, Bosi A, Barbui T. Clinical profile of homozygous JAK2V617F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007 March 22; doi: 10.1182/blood-2006-12-064287. [DOI] [PubMed] [Google Scholar]
- 17.Passamonti F, Rumi E, Pietra D, la Porta MG, Boveri E, Pascutto C, Vanelli L, Arcaini L, Burcheri S, Malcovati L, Lazzarino M, Cazzola M. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006 May 1;107(9):3676–3682. doi: 10.1182/blood-2005-09-3826. [DOI] [PubMed] [Google Scholar]
- 18.Larsen TS, Pallisgaard N, Moller MB, Hasselbalch HC. The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibrosis--impact on disease phenotype. Eur J Haematol. 2007 December;79(6):508–515. doi: 10.1111/j.1600-0609.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- 19.Prchal JF, Adamson JW, Murphy S, Steinmann L, Fialkow PJ. Polycythemia vera. The in vitro response of normal and abnormal stem cell lines to erythropoietin. J Clin Invest. 1978 April;61(4):1044–1047. doi: 10.1172/JCI109003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golde DW, Hocking WG, Koeffler HP, Adamson JW. Polycythemia: mechanisms and management. Ann Intern Med. 1981 July;95(1):71–87. doi: 10.7326/0003-4819-95-1-71. [DOI] [PubMed] [Google Scholar]
- 21.Ishii T, Bruno E, Hoffman R, Xu M. Involvement of various hematopoietic-cell lineages by the JAK2V617F mutation in polycythemia vera. Blood. 2006 November 1;108(9):3128–3134. doi: 10.1182/blood-2006-04-017392. [DOI] [PubMed] [Google Scholar]
- 22.Lacombe C, Casadevall N, Varet B. Polycythaemia vera: in vitro studies of circulating erythroid progenitors. Br J Haematol. 1980 February;44(2):189–199. doi: 10.1111/j.1365-2141.1980.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 23.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002 October 1;100(7):2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 24.Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, Score J, Seear R, Chase AJ, Grand FH, White H, Zoi C, Loukopoulos D, Terpos E, Vervessou EC, Schultheis B, Emig M, Ernst T, Lengfelder E, Hehlmann R, Hochhaus A, Oscier D, Silver RT, Reiter A, Cross NC. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005 September 15;106(6):2162–2168. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald D, Cross NC. Chronic myeloproliferative disorders: the role of tyrosine kinases in pathogenesis, diagnosis and therapy. Pathobiology. 2007;74(2):81–88. doi: 10.1159/000101707. [DOI] [PubMed] [Google Scholar]
- 26.El KN, Hetet G, Li Y, Briere J, Grandchamp B. Clonal analysis of haemopoietic cells in essential thrombocythaemia. Br J Haematol. 1995 May;90(1):131–137. doi: 10.1111/j.1365-2141.1995.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferraris AM, Mangerini R, Racchi O, Rapezzi D, Rolfo M, Casciaro S, Gaetani GF. Heterogeneity of clonal development in chronic myeloproliferative disorders. Am J Hematol. 1999 February;60(2):158–160. doi: 10.1002/(sici)1096-8652(199902)60:2<158::aid-ajh14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Gilliland DG, Blanchard KL, Levy J, Perrin S, Bunn HF. Clonality in myeloproliferative disorders: analysis by means of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1991 August 1;88(15):6848–6852. doi: 10.1073/pnas.88.15.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Kassar N, Hetet G, Briere J, Grandchamp B. Clonality analysis of hematopoiesis in essential thrombocythemia: advantages of studying T lymphocytes and platelets. Blood. 1997 January 1;89(1):128–134. [PubMed] [Google Scholar]
- 30.Kiladjian JJ, Elkassar N, Cassinat B, Hetet G, Giraudier S, Balitrand N, Conejero C, Briere J, Fenaux P, Chomienne C, Grandchamp B. Essential thrombocythemias without V617F JAK2 mutation are clonal hematopoietic stem cell disorders. Leukemia. 2006 June;20(6):1181–1183. doi: 10.1038/sj.leu.2404214. [DOI] [PubMed] [Google Scholar]
- 31.Pemmaraju N, Moliterno AR, Williams DM, Rogers O, Spivak JL. The quantitative JAK2 V617F neutrophil allele burden does not correlate with thrombotic risk in essential thrombocytosis. Leukemia. 2007 May 17; doi: 10.1038/sj.leu.2404755. [DOI] [PubMed] [Google Scholar]
- 32.Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006 September 1;108(5):1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 33.Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, Vanin EF, Bodner S, Colamonici OR, van Deursen JM, Grosveld G, Ihle JN. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998 May 1;93(3):385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 34.Eaves CJ, Eaves AC. Erythropoietin (Ep) dose-response curves for three classes of erythroid progenitors in normal human marrow and in patients with polycythemia vera. Blood. 1978 December;52(6):1196–1210. [PubMed] [Google Scholar]
- 35.Falanga A, Marchetti M, Vignoli A, Balducci D, Russo L, Guerini V, Barbui T. V617F JAK-2 mutation in patients with essential thrombocythemia: relation to platelet, granulocyte, and plasma hemostatic and inflammatory molecules. Exp Hematol. 2007 May;35(5):702–711. doi: 10.1016/j.exphem.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 36.Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, Duffy A, Boyd EM, Bench AJ, Scott MA, Vassiliou GS, Milligan DW, Smith SR, Erber WN, Bareford D, Wilkins BS, Reilly JT, Harrison CN, Green AR. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005 December 3;366(9501):1945–1953. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- 37.Najean Y, Rain JD. Treatment of polycythemia vera: the use of hydroxyurea and pipobroman in 292 patients under the age of 65 years. Blood. 1997 November 1;90(9):3370–3377. [PubMed] [Google Scholar]
- 38.Tefferi A, Gangat N, Wolanskyj AP. Management of extreme thrombocytosis in otherwise low-risk essential thrombocythemia; does number matter? Blood. 2006 October 1;108(7):2493–2494. doi: 10.1182/blood-2006-05-025544. [DOI] [PubMed] [Google Scholar]
- 39.Berk PD, Goldberg JD, Silverstein MN, Weinfeld A, Donovan PB, Ellis JT, Landaw SA, Laszlo J, Najean Y, Pisciotta AV, Wasserman LR. Increased incidence of acute leukemia in polycythemia vera associated with chlorambucil therapy. N Engl J Med. 1981 February 19;304(8):441–447. doi: 10.1056/NEJM198102193040801. [DOI] [PubMed] [Google Scholar]
- 40.Tubiana M, Flamant R, Attie E, Hayat M. A study of hematological complications occurring in patients with polycythemia vera treated with 32-P (based on a series of 296 patients) Blood. 1968 October;32(4):536–548. [PubMed] [Google Scholar]
- 41.Polycythemia vera: the natural history of 1213 patients followed for 20 years. Gruppo Italiano Studio Policitemia. Ann Intern Med. 1995 November 1;123(9):656–664. doi: 10.7326/0003-4819-123-9-199511010-00003. [DOI] [PubMed] [Google Scholar]
- 42.Segal JB, Moliterno AR. Platelet counts differ by sex, ethnicity, and age in the United States. Ann Epidemiol. 2006 February;16(2):123–130. doi: 10.1016/j.annepidem.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 43.Moliterno AR, Williams DM, Gutierrez-Alamillo LI, Salvatori R, Ingersoll RG, Spivak JL. Mpl Baltimore: A Thrombopoietin Receptor Polymorphism Associated with Thrombocytosis. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0404241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turhan AG, Humphries RK, Cashman JD, Cuthbert DA, Eaves CJ, Eaves AC. Transient suppression of clonal hemopoiesis associated with pregnancy in a patient with a myeloproliferative disorder. J Clin Invest. 1988 June;81(6):1999–2003. doi: 10.1172/JCI113549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. Association of the neutrophil JAK2V617F allele burden to disease features in 33 ET (left panels), 94 PV (center panels), and 23 PMF (right panels) patients not receiving chemotherapy. Correlations which reached statistical significance are indicated by the presence of the linear regression line, and the R and p values.
S2. Associations of the neutrophil and CD34+ cell JAK2V617F allele burdens to disease features in 50 PV patients not receiving chemotherapy. Correlations which reached statistical significance are indicated by the presence of the linear regression line, and the R and p values.


