Abstract
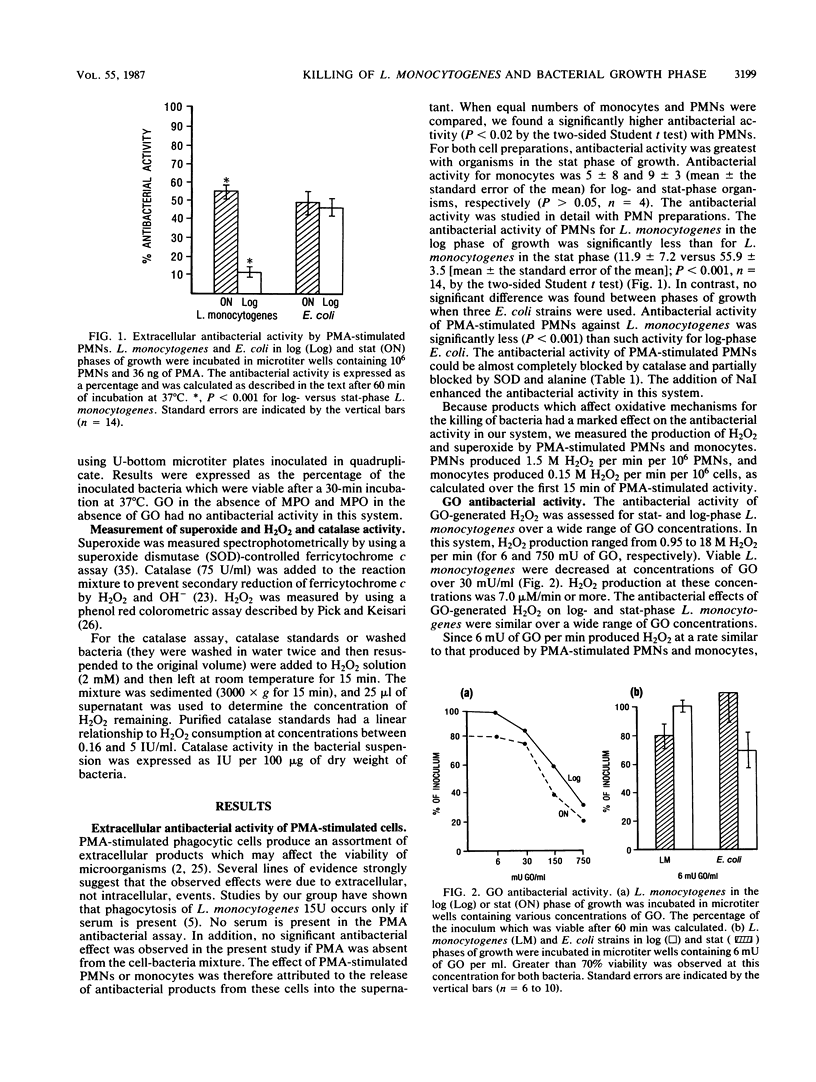
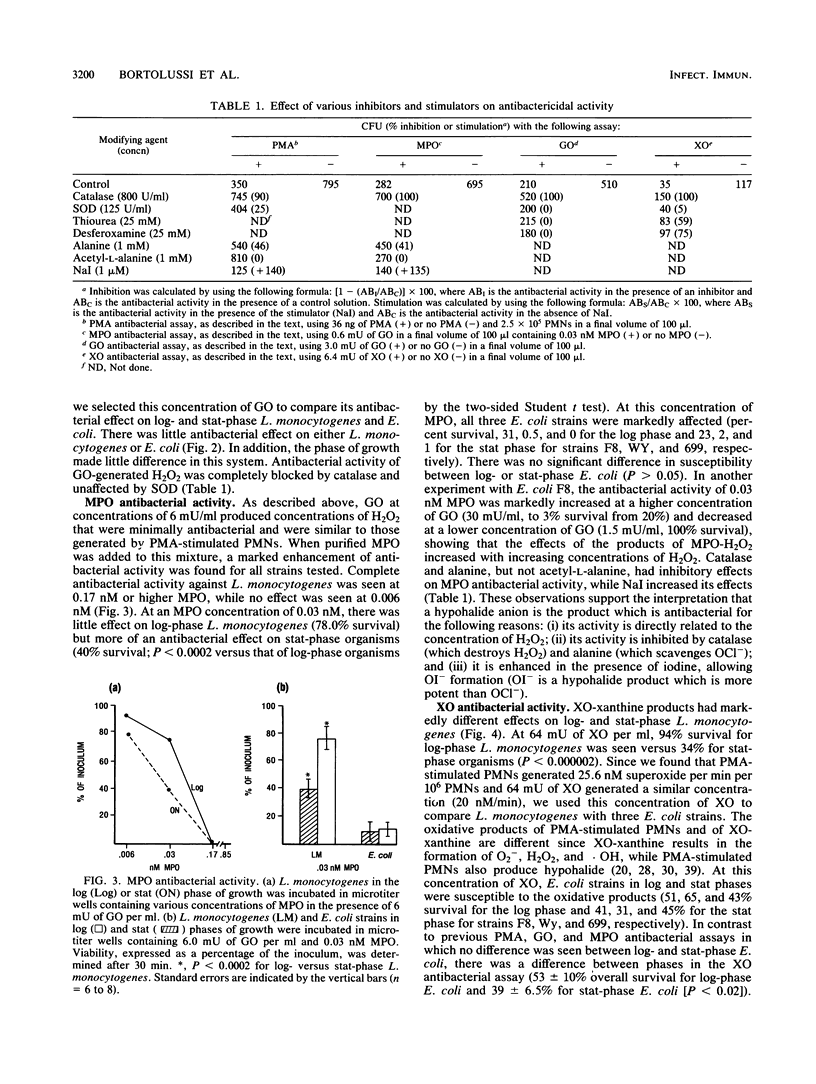
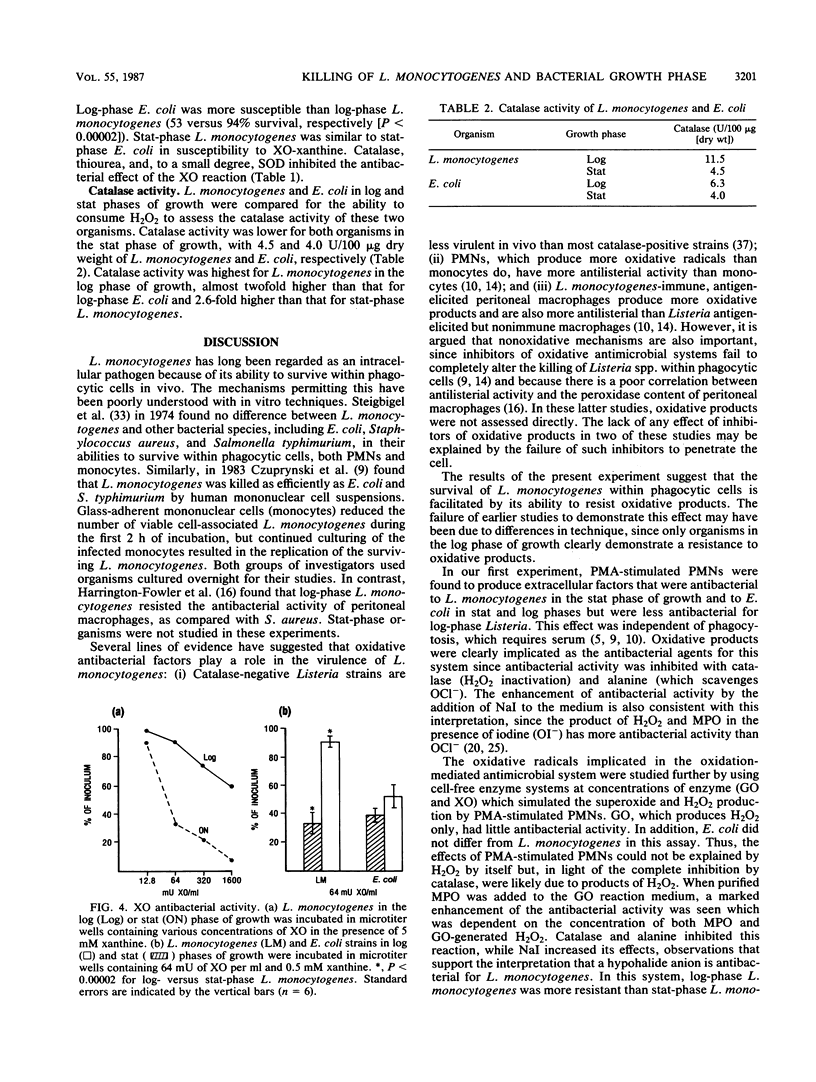
Listeria monocytogenes, a gram-positive motile bacterium which can cause severe bacterial infection in humans, is considered to be pathogenic by virtue of its ability to resist intracellular killing. Since the mechanism of intracellular survival is poorly understood, we assessed the sensitivity of L. monocytogenes to several potent antibacterial products. Phorbol myristate acetate (PMA)-stimulated polymorphonuclear cells (PMNs) produced extracellular antibacterial products which were inhibited completely by catalase, suggesting a role for oxidative agents in this process. L. monocytogenes in logarithmic (log) growth phase resisted PMA-stimulated PMN extracellular products significantly more than L. monocytogenes in stationary (stat) growth phase or Escherichia coli (three strains) in either phase of growth. The role of oxidative agents was studied further by using xanthine oxidase-xanthine, glucose oxidase-glucose, and myeloperoxidase enzyme systems to generate hydroxyl radical (.OH), hydrogen peroxide (H2O2), and hypochlorous acid (OCl-), respectively. L. monocytogenes in log phase resisted the antibacterial products of these enzyme systems under conditions which produced superoxide (O2-) and H2O2 at concentrations similar to those produced extracellularly by PMA-stimulated PMNs, while stat-growth-phase L. monocytogenes and E. coli in either phase of growth were susceptible. Antibacterial activity could be blocked or inhibited by exogenous catalase (for all oxygen radical-generating systems), mannitol, or desferoxamine (for xanthine oxidase-xanthine) and alanine (for myeloperoxidase), suggesting that .OH and OCl- were responsible for this activity. Log-phase L. monocytogenes had 2.5-fold higher bacteria-associated catalase activity, as compared with stat-phase L. monocytogenes. These experiments, therefore, suggest that log-phase L. monocytogenes resists oxidative antibacterial agents by producing sufficient catalase to inactivate these products. This may contribute to the ability of L. monocytogenes to survive intracellularly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Curnutte J. T., Kipnes R. S. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med. 1975 Feb;85(2):235–244. [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkenist A. R., Wever R., Vulsma T., Plat H., van Gelder B. F. Isolation procedure and some properties of myeloperoxidase from human leucocytes. Biochim Biophys Acta. 1978 May 11;524(1):45–54. doi: 10.1016/0005-2744(78)90101-8. [DOI] [PubMed] [Google Scholar]
- Bortolussi R., Issekutz A., Faulkner G. Opsonization of Listeria monocytogenes type 4b by human adult and newborn sera. Infect Immun. 1986 May;52(2):493–498. doi: 10.1128/iai.52.2.493-498.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Capo C., Farnarier C., Benoliel A. M., Bongrand P., Depieds R. Dissociation between phagocytosis and phagosome-lysosome fusion. J Reticuloendothel Soc. 1983 Nov;34(5):359–369. [PubMed] [Google Scholar]
- Czuprynski C. J., Campbell P. A., Henson P. M. Killing of Listeria monocytogenes by human neutrophils and monocytes, but not by monocyte-derived macrophages. J Reticuloendothel Soc. 1983 Jul;34(1):29–44. [PubMed] [Google Scholar]
- Davies W. A. Kinetics of killing Listeria monocytogenes by macrophages: correlation of 3H-DNA release from labeled bacteria and changes in numbers of viable organisms by mathematical model. J Reticuloendothel Soc. 1982 Dec;32(6):461–476. [PubMed] [Google Scholar]
- De Heer E., Kersten M. C., Van der Meer C., Linnemans W. A., Willers J. M. Electron microscopic observations on the interaction of Listeria monocytogenes and peritoneal macrophages of normal mice. Lab Invest. 1980 Nov;43(5):449–455. [PubMed] [Google Scholar]
- Evans J. R., Allen A. C., Stinson D. A., Bortolussi R., Peddle L. J. Perinatal listeriosis: report of an outbreak. Pediatr Infect Dis. 1985 May-Jun;4(3):237–241. [PubMed] [Google Scholar]
- Freund M., Pick E. The mechanism of action of lymphokines. VIII. Lymphokine-enhanced spontaneous hydrogen peroxide production by macrophages. Immunology. 1985 Jan;54(1):35–45. [PMC free article] [PubMed] [Google Scholar]
- Godfrey R. W., Wilder M. S. Relationships between oxidative metabolism, macrophage activation, and antilisterial activity. J Leukoc Biol. 1984 Oct;36(4):533–543. doi: 10.1002/jlb.36.4.533. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Swendsen C. L., Fiscus J., Miranti C. Fluorescent markers for studying phagosome-lysosome fusion. J Leukoc Biol. 1984 Sep;36(3):273–292. doi: 10.1002/jlb.36.3.273. [DOI] [PubMed] [Google Scholar]
- Harrington-Fowler L., Henson P. M., Wilder M. S. Fate of Listeria monocytogenes in resident and activated macrophages. Infect Immun. 1981 Jul;33(1):11–16. doi: 10.1128/iai.33.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Augmented induction of interferons during Listeria monocytogenes infection. J Infect Dis. 1986 May;153(5):960–969. doi: 10.1093/infdis/153.5.960. [DOI] [PubMed] [Google Scholar]
- Hawkins A. E., Bortolussi R., Issekutz A. C. In vitro and in vivo activity of various antibiotics against Listeria monocytogenes type 4b. Clin Invest Med. 1984;7(4):335–341. [PubMed] [Google Scholar]
- Ito M., Karmali R., Krim M. Effect of interferon on chemiluminescence and hydroxyl radical production in murine macrophages stimulated by PMA. Immunology. 1985 Nov;56(3):533–541. [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Mandel T. E., Cheers C. Resistance and susceptibility of mice to bacterial infection: histopathology of listeriosis in resistant and susceptible strains. Infect Immun. 1980 Dec;30(3):851–861. doi: 10.1128/iai.30.3.851-861.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah V. N., Jr, Shirley P. S., Myrvik Q., Waite M. The use of acetylated cytochrome c in detecting superoxide anion production in rabbit alveolar macrophages. J Immunol. 1983 Nov;131(5):2104–2106. [PubMed] [Google Scholar]
- Nathan C. F., Horowitz C. R., de la Harpe J., Vadhan-Raj S., Sherwin S. A., Oettgen H. F., Krown S. E. Administration of recombinant interferon gamma to cancer patients enhances monocyte secretion of hydrogen peroxide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8686–8690. doi: 10.1073/pnas.82.24.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passo S. A., Weiss S. J. Oxidative mechanisms utilized by human neutrophils to destroy Escherichia coli. Blood. 1984 Jun;63(6):1361–1368. [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Pietrangeli C. E., Skamene E., Edelson P. J., Kongshavn P. A. Measurement of 5'-nucleotidase in mouse peritoneal macrophages in listeriosis. Infect Immun. 1981 Jun;32(3):1206–1210. doi: 10.1128/iai.32.3.1206-1210.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. M., Bennedsen J., Larsen S. O., Riisgaard S., Spärck J. V. Correlation between in vivo and in vitro functional tests for activated macrophages. Infect Immun. 1979 Jan;23(1):34–40. doi: 10.1128/iai.23.1.34-40.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Oxidation of microbial iron-sulfur centers by the myeloperoxidase-H2O2-halide antimicrobial system. Infect Immun. 1985 Mar;47(3):613–618. doi: 10.1128/iai.47.3.613-618.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. Enhancement of host resistance against Listeria infection by Lactobacillus casei: role of macrophages. Infect Immun. 1984 May;44(2):445–451. doi: 10.1128/iai.44.2.445-451.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Steigbigel R. T., Lambert L. H., Jr, Remington J. S. Phagocytic and bacterial properties of normal human monocytes. J Clin Invest. 1974 Jan;53(1):131–142. doi: 10.1172/JCI107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukawa K., Mitsuyama M., Takeya K., Nomoto K. Differing contribution of polymorphonuclear cells and macrophages to protection of mice against Listeria monocytogenes and Pseudomonas aeruginosa. J Gen Microbiol. 1979 Nov;115(1):161–166. doi: 10.1099/00221287-115-1-161. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Welch D. F., Sword C. P., Brehm S., Dusanic D. Relationship between superoxide dismutase and pathogenic mechanisms of Listeria monocytogenes. Infect Immun. 1979 Mar;23(3):863–872. doi: 10.1128/iai.23.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Myeloperoxidase as an effective inhibitor of hydroxyl radical production. Implications for the oxidative reactions of neutrophils. J Clin Invest. 1986 Aug;78(2):545–550. doi: 10.1172/JCI112607. [DOI] [PMC free article] [PubMed] [Google Scholar]


