Abstract
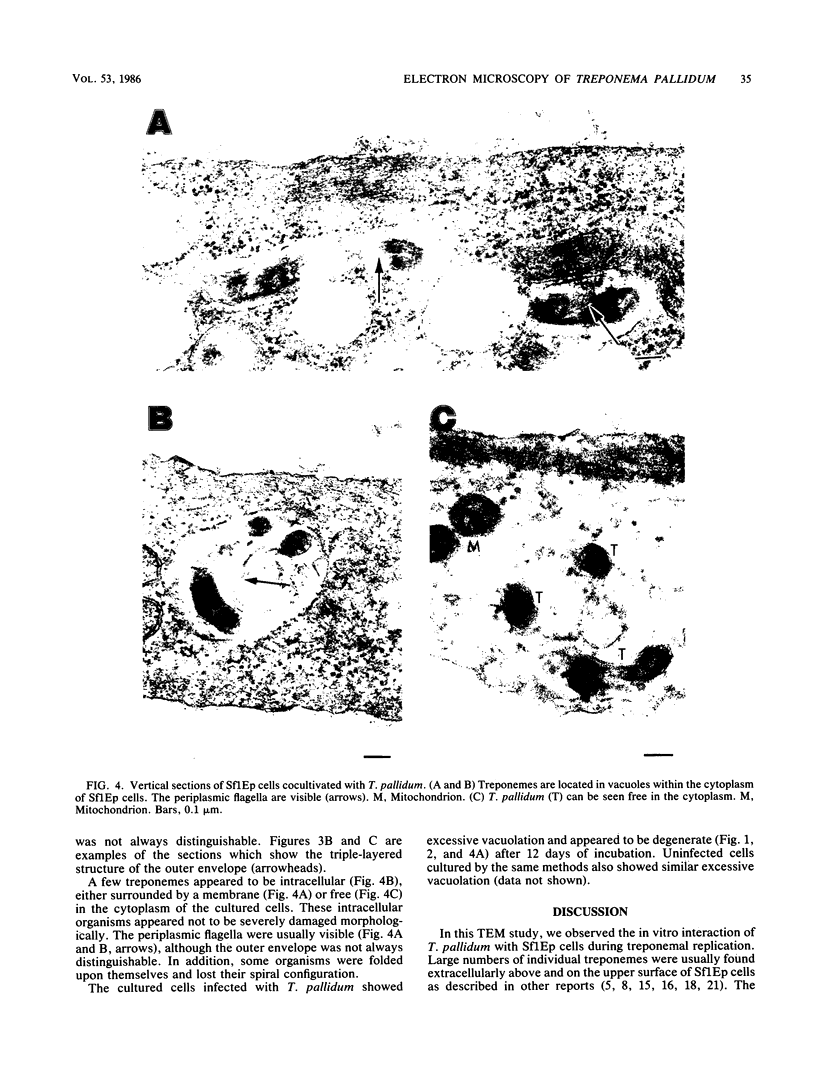
The in vitro interaction between Treponema pallidum and Sf1Ep cells during treponemal replication was investigated by using transmission electron microscopy. The Sf1Ep cells grown on Teflon-treated cover slips after 12 days of cocultivation were fixed in situ, overlaid with agar, embedded, and vertically sectioned. Large numbers of treponemes were found extracellularly not only at the upper cell surfaces but also in the narrow spaces between the cells and between the cells and the cover slips. These narrow spaces supported treponemal growth and survival, as did those at the upper cell surfaces. Although few in number, organisms were also seen in cell vacuoles either surrounded by a membrane or free in the cytoplasm. Some extracellular treponemes attached to host cells by body spirals or the terminal end and formed electron-dense layers at attachment sites. Some treponemes were often surrounded with amorphous, extracellular material which appeared to "connect" them to host cell surface. After 12 days of cocultivation, host cells showed excessive vacuolation and appeared to be damaged. This did not seem to be due to treponemal infection alone, because cells from uninfected cultures also showed similar vacuolation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Miller L. H., Johnson J., Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol. 1978 Apr;77(1):72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar H. A., Pham T. D., Kurban A. K. An electron microscopic study of a syphilitic chancre. Engulfment of Treponema pallidum by plasma cells. Arch Pathol. 1970 Aug;90(2):143–150. [PubMed] [Google Scholar]
- Chang J. P. A new technique for separation of coverglass substrate from epoxy-embedded specimens for electron microscopy. J Ultrastruct Res. 1971 Nov;37(3):370–377. doi: 10.1016/s0022-5320(71)80130-2. [DOI] [PubMed] [Google Scholar]
- Charon N. W., Lawrence C. W., O'Brien S. Movement of antibody-coated latex beads attached to the spirochete Leptospira interrogans. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7166–7170. doi: 10.1073/pnas.78.11.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Cox D. L., Moeckli R. A. Cultivation of virulent Treponema pallidum in tissue culture. Infect Immun. 1981 May;32(2):908–915. doi: 10.1128/iai.32.2.908-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Cox D. L., Moeckli R. A. Further studies on replication of virulent Treponema pallidum in tissue cultures of Sf1Ep cells. Infect Immun. 1982 Feb;35(2):449–455. doi: 10.1128/iai.35.2.449-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Cleveland P., Johnson R. C., Miller J. N., Sykes J. A. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J Bacteriol. 1977 Jun;130(3):1333–1344. doi: 10.1128/jb.130.3.1333-1344.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Gannon E. M. Further evidence for hyaluronidase activity of Treponema pallidum. Can J Microbiol. 1983 Nov;29(11):1507–1513. doi: 10.1139/m83-232. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Mucopolysaccharidase of Treponema pallidum. Infect Immun. 1979 Apr;24(1):261–268. doi: 10.1128/iai.24.1.261-268.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Ritzi D. M. Relationship of Treponema pallidum to acidic mucopolysaccharides. Infect Immun. 1979 Apr;24(1):252–260. doi: 10.1128/iai.24.1.252-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Wolff E. T. Mucopolysaccharide material resulting from the interaction of Treponema pallidum (Nichols strain) with cultured mammalian cells. Infect Immun. 1978 Nov;22(2):575–584. doi: 10.1128/iai.22.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1133–1140. doi: 10.1128/iai.11.5.1133-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Oakes S. G. Morphological destruction of cultured cells by the attachment of Treponema pallidum. Br J Vener Dis. 1982 Feb;58(1):1–11. doi: 10.1136/sti.58.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale V., Goldman J. N. Serial ultrathin sectioning demonstrating the intracellularity of T. Pallidum. An electron microscopic study. Br J Vener Dis. 1972 Apr;48(2):87–96. doi: 10.1136/sti.48.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes S. G., Repesh L. A., Pozos R. S., Fitzgerald T. J. Electrophysiological dysfunction and cellular disruption of sensory neurones during incubation with Treponema pallidum. Br J Vener Dis. 1982 Aug;58(4):220–227. doi: 10.1136/sti.58.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Further studies of the morphology of Treponema pallidum under the electron microscope. Br J Vener Dis. 1969 Jun;45(2):87–116. doi: 10.1136/sti.45.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Treponema pallidum in nerve fibres. Br J Vener Dis. 1975 Feb;51(1):10–18. doi: 10.1136/sti.51.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repesh L. A., Fitzgerald T. J., Oakes S. G., Pozos R. S. Scanning electron microscopy of the attachment of Treponema pallidum to nerve cells in vitro. Br J Vener Dis. 1982 Aug;58(4):211–219. doi: 10.1136/sti.58.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Kalan J. Intracellular Treponema pallidum in cells of a syphilitic lesion of the uterine cervix. Am J Obstet Gynecol. 1975 Jun 1;122(3):361–367. doi: 10.1016/0002-9378(75)90185-4. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N. Intracellular location of Treponema pallidum (Nichols strain) in the rabbit testis. Infect Immun. 1971 Sep;4(3):307–314. doi: 10.1128/iai.4.3.307-314.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N., Kalan A. J. Treponema pallidum within cells of a primary chancre from a human female. Br J Vener Dis. 1974 Feb;50(1):40–44. doi: 10.1136/sti.50.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzolkowa T., Kozakiewicz J. Ultrastructure of vascular and connective tissue changes in primary syphilis. Br J Vener Dis. 1980 Jun;56(3):137–143. doi: 10.1136/sti.56.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler J. A., Jones A. M., Jones R. H., Kubica K. M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976 Feb;52(1):1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]