Abstract
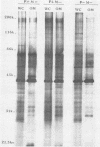
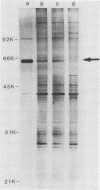
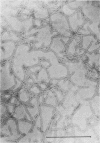
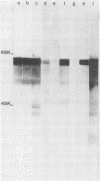
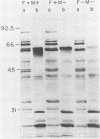
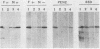
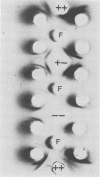
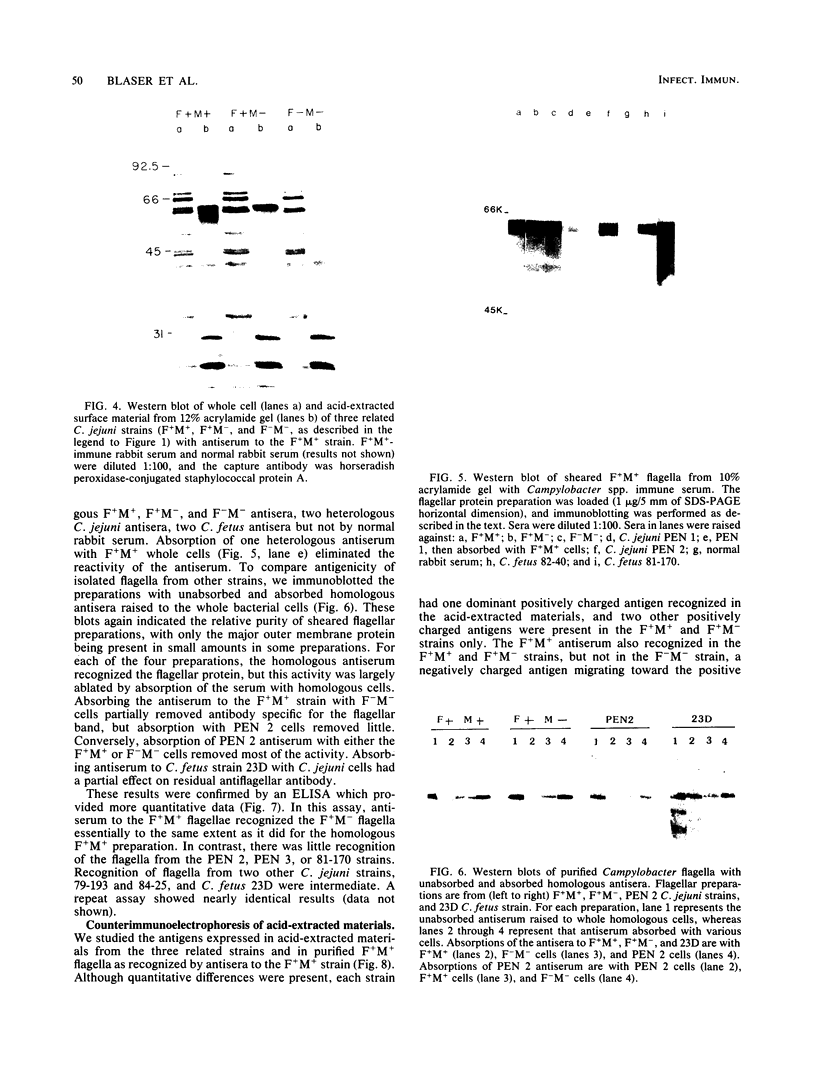
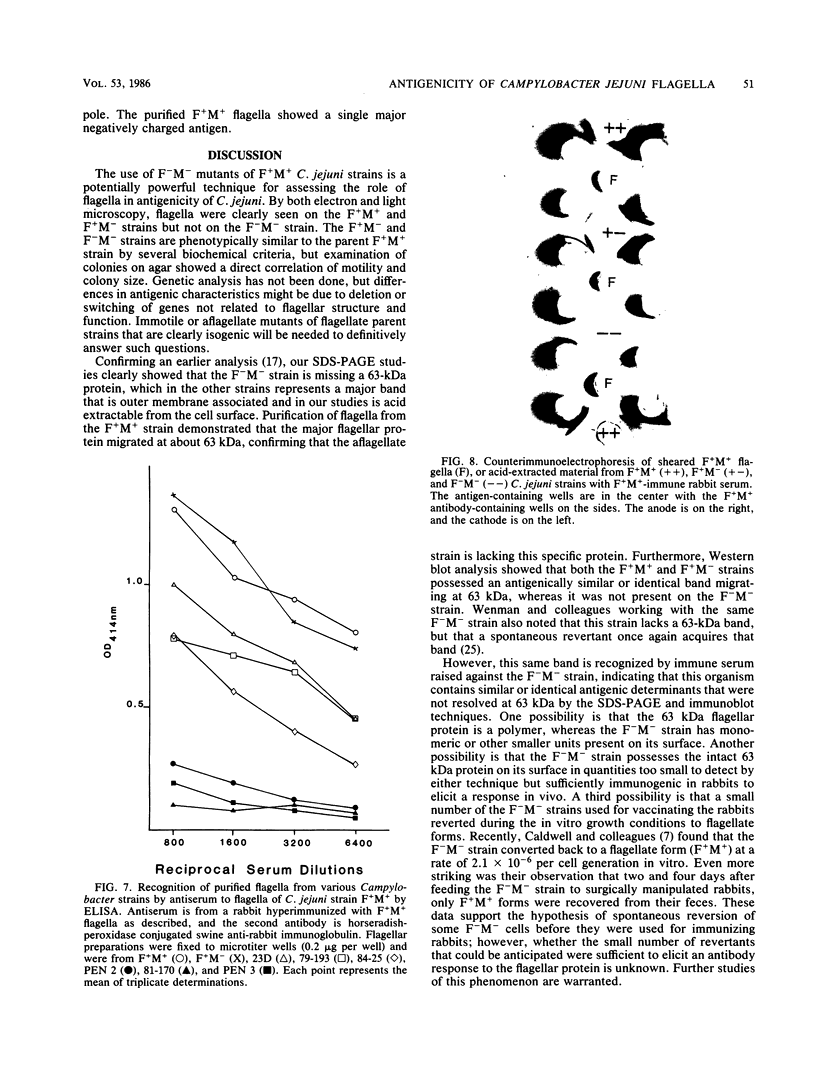
We studied the antigenicity of a wild-type flagellate and motile (F+M+) Campylobacter jejuni strain (81116) and two daughter mutants, one flagellate and immotile (F+M-) and one aflagellate and immotile (F-M-). By sodium dodecyl sulfate-polyacrylamide gel electrophoresis of acid-extracted surface proteins, a 63-kilodalton (kDa) band identified from sheared flagella as the flagellar protein was present in the F+M+ and F+M- strains but not in the F-M- strain. No other differences in protein profile among the three strains were noted. By Western blotting, serum from rabbits immunized with either the F+M+ or F-M- strain detected a 63-kDa protein in the F+M+ and F+M- strains but not in the F-M- strain. That the F-M- antiserum recognized the 63-kDa band suggests that small amounts of this protein or a cross-reacting antigen is present on the F-M- strain. By counterimmunoelectrophoresis of the acid-extracted preparations with immune sera, all three strains were found to share three major antigens, but a fourth antigen with a net positive charge was present only in the F+M+ and F+M- strains. Antisera to five C. jejuni and two Campylobacter fetus strains recognized the 63-kDa protein of purified F+M+ flagella in Western blots, demonstrating a common antigen is present, but enzyme-linked immunosorbent assay results suggest that the sharing of this antigen among Campylobacter strains is variable.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Rowley D. The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis. 1983 May;147(5):864–872. doi: 10.1093/infdis/147.5.864. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Duncan D. J. Human serum antibody response to Campylobacter jejuni infection as measured in an enzyme-linked immunosorbent assay. Infect Immun. 1984 May;44(2):292–298. doi: 10.1128/iai.44.2.292-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Vasil M. L. Campylobacter jejuni outer membrane proteins are antigenic for humans. Infect Immun. 1984 Mar;43(3):986–993. doi: 10.1128/iai.43.3.986-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hoverson D., Ely I. G., Duncan D. J., Wang W. L., Brown W. R. Studies of Campylobacter jejuni in patients with inflammatory bowel disease. Gastroenterology. 1984 Jan;86(1):33–38. [PubMed] [Google Scholar]
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Caldwell M. B., Guerry P., Lee E. C., Burans J. P., Walker R. I. Reversible expression of flagella in Campylobacter jejuni. Infect Immun. 1985 Dec;50(3):941–943. doi: 10.1128/iai.50.3.941-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Purification of intact flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):376–383. doi: 10.1128/jb.105.1.376-383.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaka H., Armfield A. Y., Lombard G. L., Dowell V. R., Jr Practical procedure for demonstrating bacterial flagella. J Clin Microbiol. 1982 Nov;16(5):948–952. doi: 10.1128/jcm.16.5.948-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983 Nov;42(2):675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McCoy E. C., Doyle D., Burda K., Corbeil L. B., Winter A. J. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect Immun. 1975 Mar;11(3):517–525. doi: 10.1128/iai.11.3.517-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montie T. C., Craven R. C., Holder I. A. Flagellar preparations from Pseudomonas aeruginosa: isolation and characterization. Infect Immun. 1982 Jan;35(1):281–288. doi: 10.1128/iai.35.1.281-288.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Pearson A. D. The identification of outer membrane proteins and flagella of Campylobacter jejuni. J Gen Microbiol. 1984 May;130(5):1201–1208. doi: 10.1099/00221287-130-5-1201. [DOI] [PubMed] [Google Scholar]
- Palmer S. R., Gully P. R., White J. M., Pearson A. D., Suckling W. G., Jones D. M., Rawes J. C., Penner J. L. Water-borne outbreak of campylobacter gastroenteritis. Lancet. 1983 Feb 5;1(8319):287–290. doi: 10.1016/s0140-6736(83)91698-7. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980 Dec;12(6):732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez G. I., Blaser M. J., Bryner J. H. Lipopolysaccharide structures of Campylobacter fetus are related to heat-stable serogroups. Infect Immun. 1986 Jan;51(1):209–212. doi: 10.21236/ada265573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez G. I., Hopkins J. A., Blaser M. J. Lipopolysaccharide structures in Enterobacteriaceae, Pseudomonas aeruginosa, and Vibrio cholerae are immunologically related to Campylobacter spp. Infect Immun. 1986 Jan;51(1):204–208. doi: 10.1128/iai.51.1.204-208.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautelin H., Kosunen T. U. An acid extract as a common antigen in Campylobacter coli and Campylobacter jejuni strains. J Clin Microbiol. 1983 Apr;17(4):700–701. doi: 10.1128/jcm.17.4.700-701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenman W. M., Chai J., Louie T. J., Goudreau C., Lior H., Newell D. G., Pearson A. D., Taylor D. E. Antigenic analysis of Campylobacter flagellar protein and other proteins. J Clin Microbiol. 1985 Jan;21(1):108–112. doi: 10.1128/jcm.21.1.108-112.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]