Abstract
Complement signaling has been implicated as important for normal hepatic regeneration. However, the specific mechanism by which complement is activated during liver regeneration remains undefined. To address this question, we investigated the hepatic regenerative response to partial hepatectomy in wildtype mice, C3-, C4-, and factor B-null mice, and C4-null mice treated with a factor B neutralizing antibody (mAb 1379). The results showed that following partial hepatectomy, C3-null mice exhibit reduced hepatic regeneration compared to wildtype mice as assessed by quantification of hepatic cyclin D1 expression and hepatocellular DNA synthesis and mitosis. In contrast, C4-null mice and factor B-null mice demonstrated normal liver regeneration. Moreover, animals in which all of the traditional upstream C3 activation pathways were disrupted, i.e. C4-null mice treated with mAb 1379, exhibited normal C3 activation and hepatocellular proliferation following partial hepatectomy. In order to define candidate non-traditional mechanisms of C3 activation during liver regeneration, plasmin and thrombin were investigated for their abilities to activate C3 in mouse plasma in vitro. The results showed that both proteases are capable of initiating C3 activation, and that plasmin can do so independent of the classical and alternative pathways.
Conclusions
These results show that C3 is required for a normal hepatic regenerative response, but that disruption of the classical- or lectin-dependent pathways (C4-dependent), the alternative pathway (factor B-dependent), or all of these pathways does not impair the hepatic regenerative response, and indicate that non-traditional mechanisms by which C3 is activated during hepatic regeneration must exist. In vitro analysis raises the possibility that plasmin may contribute to non-traditional complement activation during liver regeneration in vivo.
Keywords: Partial Hepatectomy, Alternative Pathway, Classical Pathway, Factor B, C4
Introduction
The regenerative ability of the liver is important for recovery from hepatic injury and disease. Analyses using the rodent partial hepatectomy model (Higgins and Anderson 1931) show that following partial hepatectomy, the proliferation of normally quiescent hepatocytes is rapidly induced, proceeds until the original liver mass is restored, and is then precisely terminated (Fausto 2000; Diehl and Rai 1999; Michalopoulos and DeFrances 1997). The molecular mechanisms that control this carefully orchestrated response include activation of TNFα-IL6 signaling (Akerman et al. 1992; Yamada et al. 1997; Cressman et al. 1996), generation of mitochondrial reactive oxygen species (Lee et al. 1999) and prostaglandins (Rudnick et al. 2001), and activation of stress- and mitogen-activated-protein kinase cascades (Talarmin et al. 1999). These events promote activation of NFκB, STAT3, AP1 and other transcription factors (Cressman et al. 1994; Cressman et al. 1995; Taub 1996), which direct an immediate-early gene expression program (Haber et al. 1993) culminating in growth factor-dependent, hepatocellular re-entry into and progression through the cell cycle, and restoration of normal hepatic mass. Despite the knowledge gained from experimental analyses of liver regeneration, an integrated understanding of the specific mechanisms responsible for initiation, propagation, and termination of the hepatic regenerative response remains elusive.
The complement pathway, well known for its role in host defense, as an effector arm of innate immunity, and as a regulatory element of acquired immunity (Walport 2001a, 2001b; Volanakis 1998b; Lambris et al. 2008), has recently been implicated as important in liver regeneration. This link was established by studies demonstrating that liver regeneration is impaired in complement factor C3-null mice, and that such impairment is rescued by exogenous C3 or C3a supplementation (Strey et al. 2003; Markiewski et al. 2004). Similarly, complement factor C5-deficient mice exhibit abnormal hepatic regeneration which can be rescued by supplementation with exogenous C5 or C5a (Mastellos et al. 2001; Strey et al. 2003). These analyses also show that pharmacological C5 neutralization and C5a receptor blockade disrupt the hepatic regenerative response and that disruption of complement cascade activation is associated with suppression of TNFα-IL6, NFκB, and STAT3 activation (Strey et al. 2003). Together, these data provide convincing evidence that activation of complement factor C3 and subsequent C5-dependent downstream signaling events are required for normal liver regeneration.
Although the important role of C3 and C5 signaling during liver regeneration has been established by the studies described above, the mechanisms by which such signaling is activated have not yet been defined. Complement is traditionally activated by the classical, lectin-dependent, or alternative pathway (reviewed in Lambris et al. 2008; Volanakis 1998b). The classical pathway is initiated by antibody:antigen complexes, the lectin pathway by mannan-binding lectin:carbohydrate complexes, and the alternative pathway by a variety of microbial surfaces. The alternative pathway is also activated by and can amplify the signal from the classical and lectin pathways. The classical and lectin-dependent pathways require complement factors C2 and C4 while the alternative pathway depends on complement factors B and D. Each activation pathway results in the assembly of the C3 convertases, which are the central enzymes of the complement cascade, and which catalyze the proteolytic cleavage of C3 to form C3a and C3b. C3b is an opsonin that mediates target clearance, and it can also associate with the C3 convertase to form a C5 convertase. The C5 convertase cleaves C5, forming C5a and C5b. C5b initiates the assembly of the membrane attack complex. C3a and C5a are anaphylatoxins which trigger various cellular responses via the C3a or C5a receptor, respectively.
In the studies reported here, we describe the results of our analyses of hepatic regeneration in mice disrupted for classical and lectin dependent complement activation pathways (C4-null mice), for alternative pathway activation (factor B null mice), or for both pathways (C4-null mice treated with a factor B neutralizing antibody, mAb 1379; Leinhase et al. 2007; Thurman et al. 2005, 2006; Taube et al. 2006).
Materials and Methods
Animal Husbandry and Surgery
Wildtype C57Bl/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Complement factor 3-, factor B-, and factor C4-null mice were maintained on C57Bl/6J backgrounds as previously described (Circolo et al. 1999; Matsumoto et al. 1997; Wessels et al. 1995). Absence of circulating C3, C4, or factor B in each of the respective null mice was verified by protein immunoblot performed on plasma recovered from wildtype, C3-null, C4-null, and factor B-null mice (Figure 1). One mg of the inhibitory monoclonal anti-factor B antibody mAb 1379 was administered by intraperitoneal injection once daily beginning 12 hours prior to partial hepatectomy. This is within the dosing range reported to neutralize factor B activity in models of murine inflammation (Leinhase et al. 2007; Taube et al. 2006; Thurman et al. 2005, 2006). Suppression of the complement alternative pathway in mice treated with mAb 1379 was verified using a modification of the alternative pathway zymosan assay (Thurman et al. 2005; Foley et al. 1993) as described below.
Figure 1.
Analysis of Plasma Complement Protein Levels in Wildtype and Null Mice. Protein immunoblot analysis of plasma from wildtype (WT), C3-null (C3−/−), C4-null (C4−/−), and factor B-null (fB−/−) mice for complement factor C3 (top row), C4 (middle row), or factor B (bottom row).
Two to three month old male mice, maintained on 12 h dark-light cycles and standard mouse chow, were subjected to partial hepatectomy, allowed to recover, and then sacrificed for plasma and tissue harvest as previously described (Rudnick et al. 2001; Shteyer et al. 2004; Liao et al. 2004). Briefly, mice were sedated with inhaled Isoflurane (VEDCO, Inc., St. Joseph, MO) delivered via an anesthesia cart, then subjected to mid-ventral laparotomy with exposure of the left and median hepatic lobes, which was followed by sequential ligation and resection of the median and left lobes and closure of the peritoneal and skin wounds. Hepatectomized animals were allowed to recover until the time of sacrifice by inhaled carbon dioxide and harvest of plasma and liver tissue. Three to six animals were examined at each time point and for each genotype. All experiments were approved by the Animal Studies Committee of Washington University and conducted in accordance with institutional guidelines and the criteria outlined in the “Guide for Care and Use of Laboratory Animals” (NIH publication 86-23).
Alternative Pathway Zymosan Assay
Alternative pathway integrity was measured using a modification of the zymosan assay (Thurman et al. 2005; Foley et al. 1993). Activated zymosan particles (CompTech, Tyler, TX, 1×106 per reaction) were added to 100 µl veronal buffered saline containing either 2 mM MgCl2 plus 10 mM EGTA (experimental sample), or 10 mM EDTA (negative control). Mouse plasma (10 µl) was added and the reactions were incubated at 37 degrees for 30 min. Particles were then washed twice in PBS/1% BSA, resuspended in 100 µl PBS/BSA and treated with FITC-conjugated goat anti-mouse C3 antibody (MP Biomedicals, Solon, OH; 1 µl of 1:10 dilution) at 4 degrees for 20 min. Samples were washed once and surface C3 was analyzed by FACS with a FACScaliber instrument (Becton Dickinson). A common size gate was used for all experiments. Two Mg-EGTA control reactions were performed, one without plasma and one with an unrelated FITC-conjugated antibody. Alternative pathway activity was calculated as the (Mean Particle Fluorescence of the Mg-EGTA reaction) - (Mean Particle Fluorescence of the EDTA reaction).
Histology and Immunohistochemistry
Liver histology and hepatocellular bromodeoxyuridine (BrdU) incorporation were assessed as previously described (Rudnick et al. 2001; Shteyer et al. 2004; Liao et al. 2004). Briefly, animals were injected with 100 mg/kg BrdU 1 hour prior to sacrifice. After harvesting, a portion of the right hepatic lobe was fixed in formalin, paraffin-embedded, and stained either with hematoxylin and eosin or for nuclear BrdU incorporation. The frequency of nuclear BrdU labeling was determined by two different investigators and by examination of at least three random 400x fields and at least 300 cells and nuclei in each tissue section.
Protein Expression Analysis
Whole cell lysates were made from snap frozen liver and their protein concentration determined as previously described (Rudnick et al. 2001). Twenty-five µg aliquots of protein lysate or 1 µl aliquots of plasma were subjected to SDS-PAGE, followed by electrophoretic transfer to nitrocellulose. Filters were probed with primary antibody (Cyclin D1, Upstate, Lake Placid, NY; glyceraldehyde phosphate dehydrogenase, Chemicon International, Temecula, CA; complement factor C4 and complement factor B, CompTech; complement factor C3, MP Biomedicals) followed by a horseradish peroxidase-conjugated secondary antibody, and then developed using the ECL system (Amersham, Piscataway, NJ). Densitometric analysis was performed with Scion Image data analysis software (Scion Corporation, Frederick, MD).
In Vivo and In Vitro Analysis of C3 Activation
Activation of C3 during liver regeneration was evaluated by protein immunoblot determination of the activated ~40 kDa C3α proteolytic fragment in plasma as previously described (Mastellos et al. 2004; Miwa et al. 2007). In vitro activation of C3 was determined using this same methodology on mouse plasma incubated with thrombin (Sigma Chemical Company, St. Louis; 18 units to 10 µl plasma) or plasmin (Sigma Chemical Company; 10 µg, ≥0.03 units, added to 10 µl of plasma), at 25° C for 20 minutes.
Statistical Analysis
Data were analyzed using SigmaPlot and SigmaStat software (SPSS, Chicago, IL). Unpaired Student’s t-test for pair-wise comparisons and ANOVA for multiple groups (with the Holm-Sidak method for post-hoc comparison) were used to determine statistical significance for differences in hepatocellular BrdU incorporation, mitotic body frequency, and protein expression. Kaplan-Meier survival analysis was performed using the LogRank statistic to compare outcomes between groups. Data are reported as mean ± standard error.
Results
Liver Regeneration is Delayed in C3-Null Mice
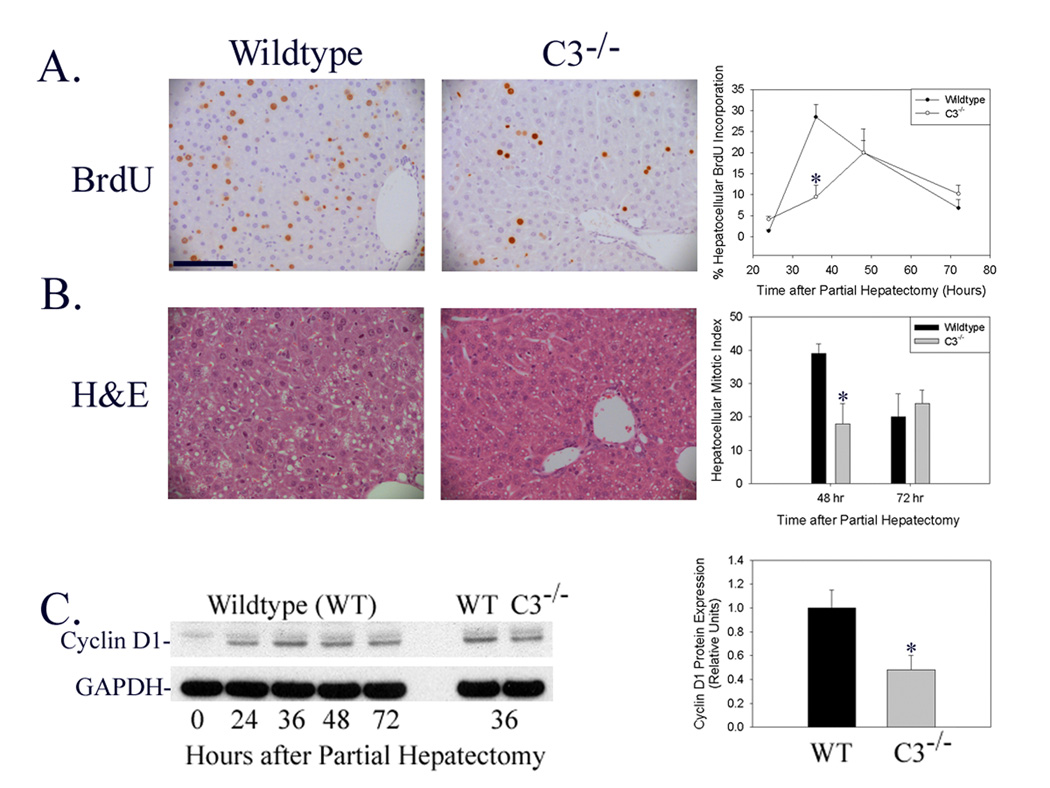
Determination of hepatocellular BrdU incorporation following partial hepatectomy in wildtype and C3-null mice showed that hepatocellular proliferation was inhibited and the time to peak proliferation delayed in C3-null mice (Figure 2A, *p<0.05). Similarly, hepatocellular mitotic body frequency was reduced in C3-null mice at 48 hours after surgery (Figure 2B, *p<0.02), which is the timepoint corresponding to peak hepatocellular mitosis in wildtype mice (Liao et al. 2004). Finally, induction of hepatic cyclin D1 protein expression, which regulates hepatocellular G1-S phase cell cycle progression during normal regeneration, was suppressed in C3-null mice (Figure 2C: Immunoblot representative of n=6 animals for each timepoint shown on left; densitometric analysis of individual data from all animals summarized on right; *p<0.03). Together these data indicate that liver regeneration is delayed by genetic disruption of complement factor C3 expression. This delay was not associated with histological evidence of increased liver tissue injury (Figure 2B) or with increased animal mortality from 0–72 hours after surgery.
Figure 2.
Liver Regeneration in Wildtype and C3-Null Mice. (A) Immunohistochemical analysis of hepatocellular BrdU incorporation 36 hours after partial hepatectomy (left and middle panel) and summary of hepatocellular proliferation (fraction of total hepatocytes that incorporated BrdU) 24–72 hours after partial hepatectomy (right panel) in regenerating liver harvested from wildtype and C3-null (C3−/−) mice. (100 micron scale bar shown in upper left panel.) (B) Hematoxylin and eosin (H&E) stained sections of liver harvested 48 hours after partial hepatectomy (left and middle panels) and summary of hepatocellular mitotic index (right panel, mitoses per ten x200 fields) in regenerating liver harvested from wildtype and C3-null mice. (C) Representative protein immunoblot (left panel) and densitometric (right panel) analysis of hepatic cyclin D1 expression at serial times after partial hepatectomy in wildtype mice and at 36 hours after partial hepatectomy in wildtype and C3-null mice. Immunoblot analysis for glyceraldehyde phosphate dehydrogenase (GAPDH) is shown as loading control.
Liver Regeneration Occurs Normally in C4-Null Mice
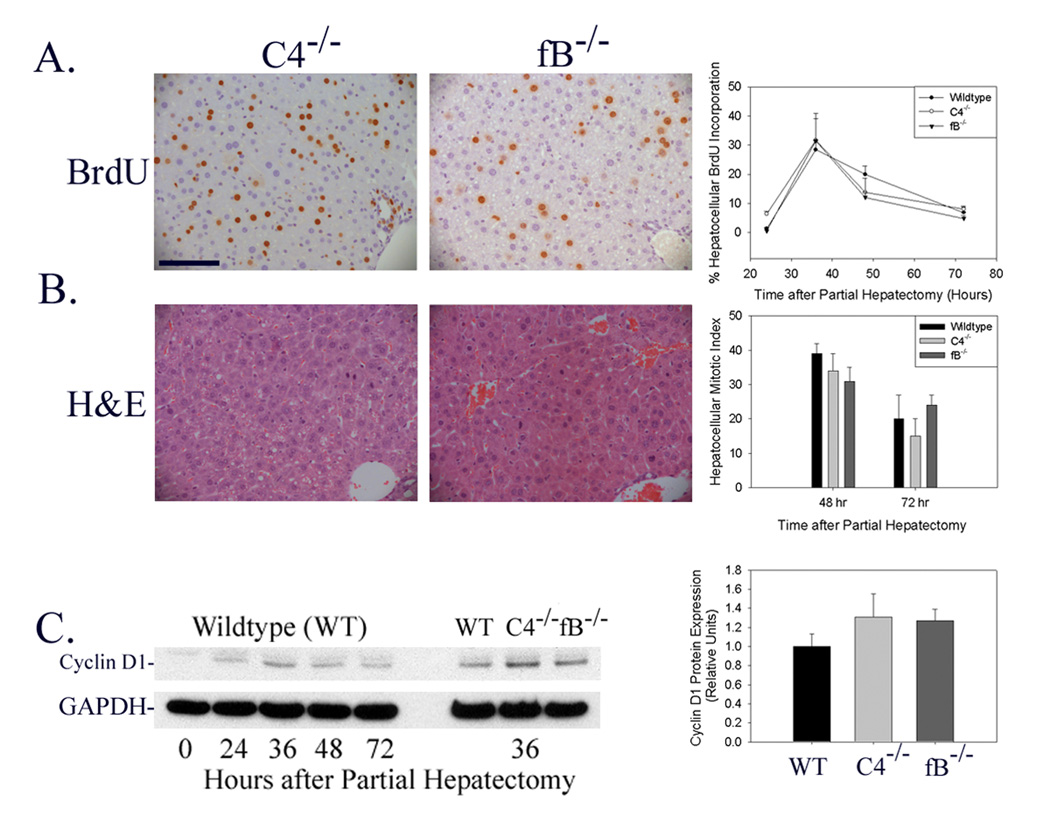
In order to determine whether either the complement classical or lectin activation pathways are required for normal liver regeneration, the hepatic regenerative response was evaluated in complement factor C4-null mice. C4 is a necessary component of the classical and lectin-dependent pathway C3- and C5-convertases (Volanakis 1998a). Analysis of the regenerative response to partial hepatectomy in C4-null mice showed that hepatocellular BrdU incorporation, mitotic body frequency, and cyclin D expression were not inhibited or delayed by genetic disruption of C4 expression (Figure 3A–C; Immunoblot shown in Figure 3C is representative of n=6 animals for each timepoint shown on left; densitometric analysis of individual data from all animals summarized on right). In addition, there was no evidence for increased liver tissue injury (Figure 3B) or increased animal mortality in C4-null mice subjected to partial hepatectomy. These data indicate that neither the classical nor the lectin-dependent pathways of complement cascade activation are absolutely required for normal hepatic regeneration.
Figure 3.
Liver Regeneration in C4-Null and Factor B-Null Mice. (A) Immunohistochemical analysis of hepatocellular BrdU incorporation 36 hours after partial hepatectomy (left and middle panel) and summary of hepatocellular proliferation (fraction of total hepatocytes that incorporated BrdU) 24–72 hours after partial hepatectomy (right panel) in regenerating liver harvested from C4-null (C4−/−) and factor B-null (fB−/−) mice. (100 micron scale bar shown in upper left panel.) (B) Hematoxylin and eosin (H&E) stained sections of liver harvested 48 hours after partial hepatectomy (left and middle panels) and summary of hepatocellular mitotic index (right panel, mitoses per ten x200 fields) in regenerating liver harvested from C4- and factor B-null mice. (C) Representative protein immunoblot (left panel) and densitometric (right panel) analysis of hepatic cyclin D1 expression at serial times after partial hepatectomy in wildtype mice and at 36 hours after partial hepatectomy in wildtype, C4-null, and factor B-null mice. Immunoblot analysis for glyceraldehyde phosphate dehydrogenase (GAPDH) is shown as loading control.
Liver Regeneration Occurs Normally in Factor B-Null Mice
Next, in order to determine whether the complement alternative activation pathway is required during liver regeneration, the hepatic regenerative response to partial hepatectomy was quantified in complement factor B-null mice. A major fragment of factor B is a component of the alternative pathway C3- and C5-convertases (Volanakis 1998a). Moreover, the alternative pathway has been shown, in most instances, to play an obligate role in generating complement activation products (Thurman and Holers 2006). Analysis of the regenerative response to partial hepatectomy in factor B-null mice showed that hepatocellular BrdU incorporation, cyclin D expression, and mitotic body frequency were not inhibited or delayed by genetic disruption of factor B expression (Figure 3A–C; Immunoblot shown in Figure 3C is representative of n=6 animals for each timepoint shown on left; densitometric analysis of individual data from all animals summarized on right). As was true for C3- and C4-null mice, there was no evidence of increased liver tissue injury or increased mortality in factor B null mice subjected to partial hepatectomy. These data show that the alternative pathway of complement cascade activation is not required for normal hepatic regeneration.
Liver Regeneration and C3 Activation Occur Normally in C4-Null Mice Treated with mAb 1379 Factor B Neutralizing Antibody
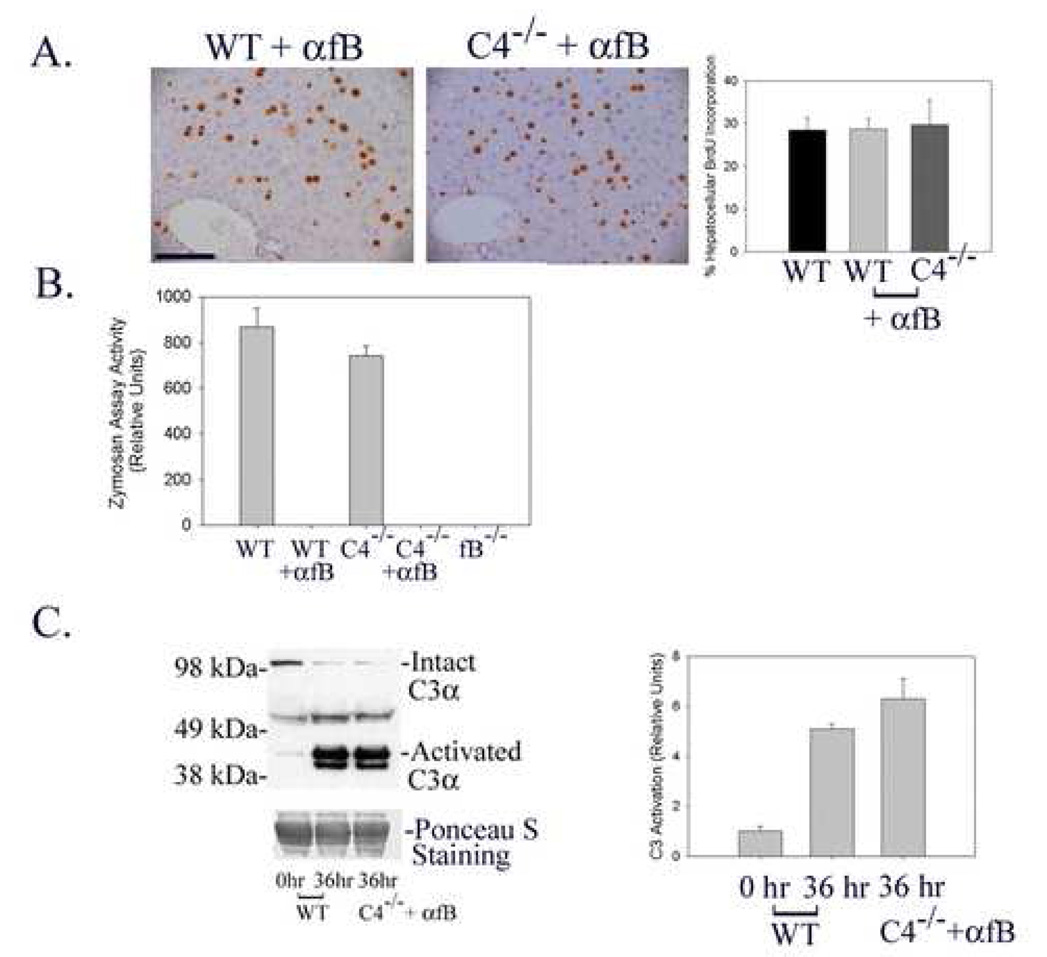
The data described above indicate that neither the classical, lectin-dependent, nor alternative pathways of complement activation are absolutely required for normal liver regeneration. However, these analyses do not distinguish between the possibilities that any of these traditional upstream pathways may be sufficient to activate C3 and promote normal liver regeneration or, alternatively, that non-traditional mechanisms activate complement signaling during liver regeneration. To investigate these considerations, the hepatic regenerative response was evaluated in C4-null mice treated with a well characterized anti-factor B neutralizing monoclonal antibody (mAb 1379, (Leinhase et al. 2007; Taube et al. 2006; Thurman et al. 2005, 2006)). In these animals, the classical and lectin-dependent pathways are blocked by genetic disruption of C4 expression (Figure 1) while the alternative pathway is entirely suppressed by the neutralizing antibody (Figure 4B). The results showed that hepatocellular proliferation 36 hours after partial hepatectomy, the time corresponding to peak proliferation in wildtype mice, is comparable in wildtype untreated-, wildtype mAb 1379-treated-, and C4 null mAb 1379-treated mice (Figure 4A), indicating that disruption of all of the traditional upstream activation pathways of complement signaling does not prevent normal liver regeneration. Taken together with the observation that complement factor C3 is required for normal liver regeneration (Mastellos et al. 2001;Strey et al. 2003;Markiewski et al. 2004), these data raise the possibility that C3 is activated independently of the classical-, lectin-dependent-, and alternative-pathways during the hepatic regenerative response. To test this possibility, C3 activation during liver regeneration was quantified in wildtype and mAb 1379-treated C4 null mice subjected to partial hepatectomy by protein immunoblot analysis of mouse plasma for the proteolytic fragment corresponding to activated C3α (Miwa et al. 2007). The results showed comparable C3 activation in each case (Figure 4C; Immunoblot shown on left is representative of n=3 animals for each timepoint and treatment group; densitometric analysis of individual data from all animals summarized on right), indicating that C3 must be activated by non-traditional mechanisms during liver regeneration.
Figure 4.
Liver Regeneration in Wildtype and C4-null mAb 1379-Treated Mice. (A) Immunohistochemical analysis (left and middle panels) and summary (right panel) of hepatocellular proliferation (fraction of total hepatocytes that incorporated BrdU) 36 hours after partial hepatectomy in regenerating liver from wildtype (WT) and C4-null (C4−/−) mice treated with factor B neutralizing antibody (αfB, mAb 1379). (100 micron scale bar shown in left panel.) (B) Determination of alternative pathway activity by zymosan assay analysis of plasma recovered 36 hours after partial hepatectomy from wildtype and C4-null mice in the absence and presence of mAb 1379 and in factor B-null (fB−/−) mice. (C) Representative protein immunoblot (left panel) and densitometric (right panel) analysis of complement C3 activation in plasma recovered 36 hours after partial hepatectomy from wildtype and C4-null mice treated with mAb 1379. Plasma from un-operated wildtype mice is shown as control, and Ponceau S staining is shown as loading control.
In Vitro Proteolytic Activation of C3
Based on the data described above and a recent report demonstrating non-traditional (C3-independent) proteolytic activation of complement factor C5 by thrombin (Huber-Lang et al. 2006), the effect of thrombin on in vitro C3 activation was assessed by incubating purified protease with wildtype mouse plasma. The results showed thrombin-dependent appearance of activated C3α (Figure 5, lanes a–c; The immunoblot shown is representative of an experiment performed in duplicate). The protease plasmin, which is an important regulator of liver regeneration (Drixler et al. 2003), was also investigated, and similar results were seen (Figure 5, lanes a–b,d). Further analysis showed that plasmin dependent C3 activation was comparable in wildtype, factor B null-, C4 null-, and mAb 1379 treated C4 null-mice (Figure 5, lanes d,h,l,p) whereas thrombin dependent activation was reduced in plasma from factor B null- and mAb 1379 treated C4 null-mice (Figure 5, lanes c,g,k,o). These data show that under these experimental conditions plasmin can promote C3 activation independent of the alternative, classical, and lectin dependent pathways in vitro. This observation raises the possibility that plasmin may contribute to non-traditional complement activation during liver regeneration in vivo.
Figure 5.
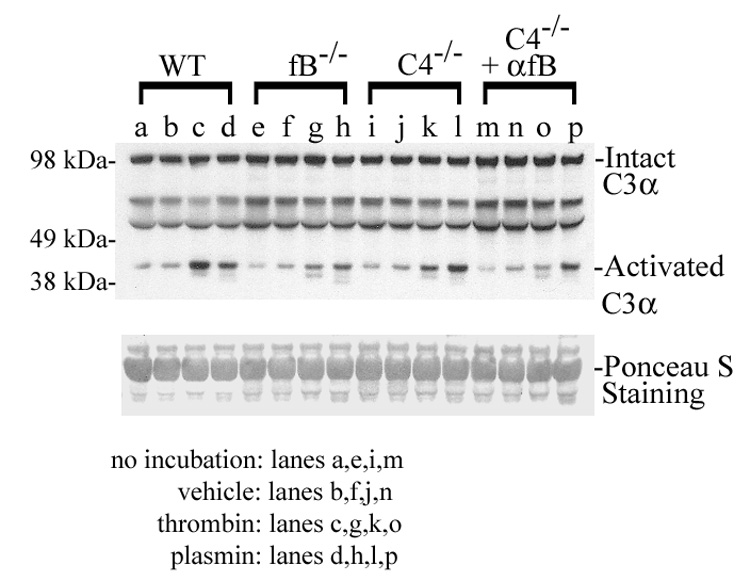
Representative protein immunoblot analysis of complement C3 activation in vitro in plasma from wildtype (WT), factor B null (fB−/−), C4 null (C4−/−), and mAb 1379 treated C4 null (C4−/−+αfB) mice unincubated (lanes a,e,i,m), or incubated in the absence (lanes b,f,j,n) or presence of thrombin (lanes c,g,k,o) or plasmin (lanes d,h,l,p). Ponceau S staining is shown as loading control.
Discussion and Conclusions
The complement system is well known for its immunological role in defense against invading pathogens, and has also been implicated in the pathogenesis of autoimmune and other diseases in which tissue injury results from dysregulated complement-dependent release of inflammatory mediators (reviewed in Volanakis 1998b; Lambris et al. 2008). More recent evidence indicates that complement may also be an important regulator of various developmental processes including bone and cartilage growth, reproduction, hematopoiesis, vascular development, and tissue and organ regeneration (Mastellos and Lambris 2002). Complement activation during host defense traditionally occurs through one of three pathways: the classical-, lectin-dependent, or alternative pathways (reviewed in Walport 2001a, 2001b; Volanakis 1998b). The classical and lectin-dependent pathways require complement factors C2 and C4 while the alternative pathway depends on complement factors B and D. The regulation of complement activation during various developmental processes is not as well established. For example, although complement signaling is known to be required for regeneration of the newt limb (Kimura et al. 2003; Rio-Tsonis et al. 1998) and the mammalian liver (Mastellos et al. 2001; Strey et al. 2003; Markiewski et al. 2004), the events that regulate complement cascade activation in these settings have not yet been defined. Therefore, in the analyses reported here, we verified the role of C3 and assessed the functional requirement for each of the known upstream complement activation pathways during liver regeneration.
Our data show that C3-null mice exhibit impaired hepatic regeneration characterized by significantly delay in hepatocellular proliferation, mitotic progression, and cyclin D expression following partial hepatectomy (Figure 1). Although these observations are consistent with the previous report showing that C3 is required for normal liver regeneration (Strey et al. 2003), the phenotype in C3-null mice reported here is not as severe, with decreased tissue injury and mortality, compared to that described in the prior study. The basis for this difference is not known, but is suspected to reflect technical differences in application of the surgical model: the partial hepatectomy methodology used here has been modified from that reported by Higgins and Anderson (Higgins and Anderson 1931) to incorporate use of controlled vaporizer-based delivery of inhaled Isoflurane anesthesia, a change which has resulted in diminished morbidity and mortality in this model system (personal observations of Clark, Weymann, and Rudnick). Nevertheless, our data support the conclusion that complement factor C3 is required for normal liver regeneration.
The data described here also show that disruption of specific factors required for the classical and lectin activation pathways (C4 null mice) or for the alternative pathway (factor B null mice) does not impair hepatocellular proliferation following partial hepatectomy. With respect to C4 disruption, two complement activation mechanisms involving classical or lectin pathway components that bypass the standard C4 requirement have been reported: Antibody-antigen complexes, which normally activate the classical pathway, and mannose-binding-lectin:lectin complexes, which normally activate the lectin pathway are both capable of activating C3 in the absence of C4 (Atkinson and Frank 2006; Selander et al. 2006). However, in each case the alternative pathway plays a critical role. In contrast, our data demonstrate that liver regeneration and C3 activation are also normal in C4 null mice treated with the factor B-neutralizing antibody (Figure 4).
Taken together, the data reported here indicate that despite the established requirement for complement factors C3 and C5 during the hepatic regenerative response (Mastellos et al. 2001; Strey et al. 2003; Markiewski et al. 2004), none of the traditional upstream complement activation pathways are absolutely necessary for normal liver regeneration. The most straightforward interpretation of these data is that proteolytic C3 activation during liver regeneration may occur via non-traditional mechanisms. This conclusion is particularly intriguing in light of a recently published report demonstrating non-traditional (C3-independent) activation of C5 by thrombin both in vivo in a murine model of lung injury and in vitro (Huber-Lang et al. 2006), and raise the possibility that thrombin or other factors may also promote C3 activation during liver regeneration. Based on such analyses and recently reported observations highlighting the existence of crosstalk between the complement and coagulation proteolytic cascades (Markiewski et al. 2007), the effects of thrombin and plasmin on in vitro C3 activation were determined. The results showed that both of these proteases can promote the generation of activated C3α, and furthermore that under the experimental conditions employed here plasmin can promote such activation in the absence of factor B (Figure 5). Together, our data raise the possibility that non-traditional mechanisms involving plasmin or other proteases may promote complement factor C3 activation during normal liver regeneration in vivo. Future studies should investigate the extent to which such mechanisms contribute to complement function in liver regeneration and other settings.
Acknowledgements
We are grateful to Drs. Harvey Colten and Hector Molina for providing the C3-null and factor B-null mice, to Lynn Mitchell for technical assistance with the zymosan assay, and to Dr. Xiaobo Wu for assistance with the complement factor C3 activation immunoblot analysis. The studies reported here were supported by grants to DAR from NIH (DK068219) and March of Dimes (Basil O’Connor Award), to DH from NIH (AI05143), and by the Digestive Disease Research Core Center (NIH grant # P30 DK52574). AW was supported in part by a post-doctoral fellowship award from the American Liver Foundation. YPT was supported in part by Institutional Training Grant T32-HD07409. JMT is supported by NIH DK064790.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. American Journal of Physiology. 1992;263:G579–G585. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson JP, Frank MM. Bypassing complement: evolutionary lessons and future implications. J.Clin.Invest. 2006;116:1215–1218. doi: 10.1172/JCI28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Circolo A, Garnier G, Fukuda W, Wang X, Hidvegi T, Szalai AJ, Briles DE, Volanakis JE, Wetsel RA, Colten HR. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology. 1999;42:135–149. doi: 10.1016/s0162-3109(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 4.Cressman DE, Diamond RH, Taub R. Rapid activation of the STAT3 transcription complex in liver regeneration. Hepatology. 1995;21:1443–1449. [PubMed] [Google Scholar]
- 5.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 6.Cressman DE, Greenbaum LE, Haber BA, Taub R. Rapid activation of post-hepatectomy factor/nuclear factor kappa B in hepatocytes, a primary response in the regenerating liver. J.Biol.Chem. 1994;269:30429–30435. [PubMed] [Google Scholar]
- 7.Diehl AM, Rai R. Liver Regeneration. In: Schiff R, Sorrell MF, Maddrey WC, editors. Schiff's Diseases of the Liver. Philadelphia: Lippincott-Raven; 1999. pp. 39–54. [Google Scholar]
- 8.Drixler TA, Vogten JM, Gebbink MF, Carmeliet P, Voest EE, Borel R, I Plasminogen mediates liver regeneration and angiogenesis after experimental partial hepatectomy. British Journal of Surgery. 2003;90:1384–1390. doi: 10.1002/bjs.4275. [DOI] [PubMed] [Google Scholar]
- 9.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 10.Foley S, Li B, Dehoff M, Molina H, Holers VM. Mouse Crry/p65 is a regulator of the alternative pathway of complement activation. European Journal of Immunology. 1993;23:1381–1384. doi: 10.1002/eji.1830230630. [DOI] [PubMed] [Google Scholar]
- 11.Haber BA, Mohn KL, Diamond RH, Taub R. Induction patterns of 70 genes during nine days after hepatectomy define the temporal course of liver regeneration. J.Clin.Invest. 1993;91:1319–1326. doi: 10.1172/JCI116332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins GM, Anderson RM. Experimental Pathology of the Liver. 1. Restoration of the liver of the white rat following partial surgical removal. Arch.Pathol. 1931;12:186–202. [Google Scholar]
- 13.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat.Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 14.Kimura Y, Madhavan M, Call MK, Santiago W, Tsonis PA, Lambris JD, Rio-Tsonis K. Expression of complement 3 and complement 5 in newt limb and lens regeneration. J.Immunol. 2003;170:2331–2339. doi: 10.4049/jimmunol.170.5.2331. [DOI] [PubMed] [Google Scholar]
- 15.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat.Rev.Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee FY, Li Y, Zhu H, Yang S, Lin HZ, Trush M, Diehl AM. Tumor necrosis factor increases mitochondrial oxidant production and induces expression of uncoupling protein-2 in the regenerating mice [correction of rat] liver. Hepatology. 1999;29:677–687. doi: 10.1002/hep.510290320. [DOI] [PubMed] [Google Scholar]
- 17.Leinhase I, Rozanski M, Harhausen D, Thurman JM, Schmidt OI, Hossini AM, Taha ME, Rittirsch D, Ward PA, Holers VM, Ertel W, Stahel PF. Inhibition of the alternative complement activation pathway in traumatic brain injury by a monoclonal anti-factor B antibody: a randomized placebo-controlled study in mice. J.Neuroinflammation. 2007;4:13. doi: 10.1186/1742-2094-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao Y, Shikapwashya ON, Shteyer E, Dieckgraefe BK, Hruz PW, Rudnick DA. Delayed hepatocellular mitotic progression and impaired liver regeneration in early growth response-1-deficient mice. J.Biol.Chem. 2004;279:43107–43116. doi: 10.1074/jbc.M407969200. [DOI] [PubMed] [Google Scholar]
- 19.Markiewski MM, Mastellos D, Tudoran R, DeAngelis RA, Strey CW, Franchini S, Wetsel RA, Erdei A, Lambris JD. C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. Journal of Immunology. 2004;173:747–754. doi: 10.4049/jimmunol.173.2.747. [DOI] [PubMed] [Google Scholar]
- 20.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Mastellos D, Lambris JD. Complement: more than a 'guard' against invading pathogens? Trends in Immunology. 2002;23:485–491. doi: 10.1016/s1471-4906(02)02287-1. [Review] [36 refs] [DOI] [PubMed] [Google Scholar]
- 22.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J.Immunol. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 23.Mastellos D, Prechl J, Laszlo G, Papp K, Olah E, Argyropoulos E, Franchini S, Tudoran R, Markiewski M, Lambris JD, Erdei A. Novel monoclonal antibodies against mouse C3 interfering with complement activation: description of fine specificity and applications to various immunoassays. Mol.Immunol. 2004;40:1213–1221. doi: 10.1016/j.molimm.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin DD, Colten HR. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8720–8725. doi: 10.1073/pnas.94.16.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [Review] [DOI] [PubMed] [Google Scholar]
- 26.Miwa T, Zhou L, Tudoran R, Lambris JD, Madaio MP, Nangaku M, Molina H, Song WC. DAF/Crry double deficiency in mice exacerbates nephrotoxic serum-induced proteinuria despite markedly reduced systemic complement activity. Mol.Immunol. 2007;44:139–146. doi: 10.1016/j.molimm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Rio-Tsonis K, Tsonis PA, Zarkadis IK, Tsagas AG, Lambris JD. Expression of the third component of complement, C3, in regenerating limb blastema cells of urodeles. Journal of Immunology. 1998;161:6819–6824. [PubMed] [Google Scholar]
- 28.Rudnick DA, Perlmutter DH, Muglia LJ. Prostaglandins are required for CREB activation and cellular proliferation during liver regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8885–8890. doi: 10.1073/pnas.151217998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selander B, Martensson U, Weintraub A, Holmstrom E, Matsushita M, Thiel S, Jensenius JC, Truedsson L, Sjoholm AG. Mannan-binding lectin activates C3 and the alternative complement pathway without involvement of C2. J.Clin.Invest. 2006;116:1425–1434. doi: 10.1172/JCI25982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shteyer E, Liao Y, Muglia LJ, Hruz PW, Rudnick DA. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology. 2004;40:1322–1332. doi: 10.1002/hep.20462. [DOI] [PubMed] [Google Scholar]
- 31.Strey CW, Markiewski M, Mastellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. Journal of Experimental Medicine. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, Loyer P, Guguen-Guillouzo C, Baffet G. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signalling pathway involved in the regulation of G(1) phase progression in proliferating hepatocytes. Molecular & Cellular Biology. 1999;19:6003–6011. doi: 10.1128/mcb.19.9.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taub R. LIVER REGENERATION .4. Transcriptional control of liver regeneration. FASEB Journal. 1996;10:413–427. [PubMed] [Google Scholar]
- 34.Taube C, Thurman JM, Takeda K, Joetham A, Miyahara N, Carroll MC, Dakhama A, Giclas PC, Holers VM, Gelfand EW. Factor B of the alternative complement pathway regulates development of airway hyperresponsiveness and inflammation. Proc.Natl.Acad.Sci.U.S.A. 2006;103:8084–8089. doi: 10.1073/pnas.0602357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J.Immunol. 2006;176:1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 36.Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, Mitchell LM, Giclas PC, Salmon J, Gilkeson G, Holers VM. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol.Immunol. 2005;42:87–97. doi: 10.1016/j.molimm.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 37.Thurman JM, Royer PA, Ljubanovic D, Dursun B, Lenderink AM, Edelstein CL, Holers VM. Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J.Am.Soc.Nephrol. 2006;17:707–715. doi: 10.1681/ASN.2005070698. [DOI] [PubMed] [Google Scholar]
- 38.Volanakis JE. In: The Human Complement System in Health and Disease. Volanakis JE, Frank MM, editors. New York: Marcel Dekker, Inc; 1998a. pp. 149–166. [Google Scholar]
- 39.Volanakis JE. Overview of the Complement System. In: Volanakis JE, Frank MM, editors. The Human Complement System in Health and Disease. New York: Marcel Dekker, Inc; 1998b. pp. 9–32. [Google Scholar]
- 40.Walport MJ. Complement. First of two parts. N.Engl.J.Med. 2001a;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 41.Walport MJ. Complement. Second of two parts. N.Engl.J.Med. 2001b;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 42.Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc.Natl.Acad.Sci.U.S.A. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]