Abstract
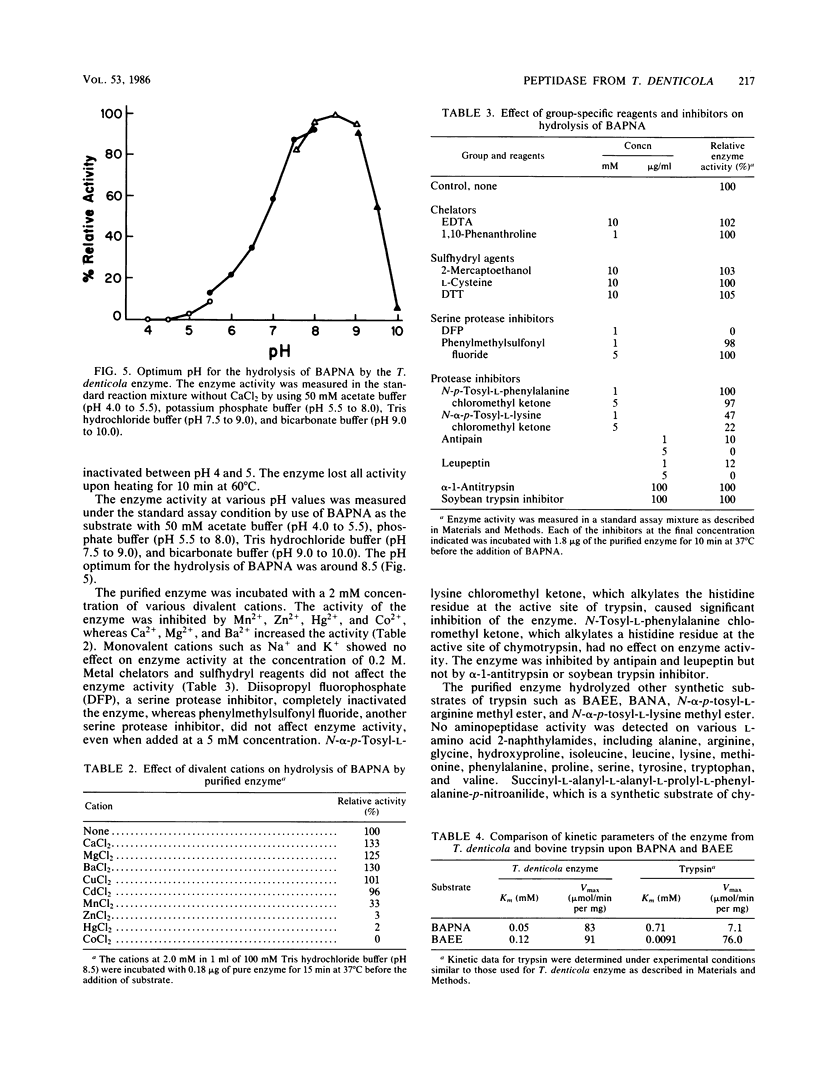
An enzyme from Treponema denticola that hydrolyzes a synthetic trypsin substrate, N-alpha-benzoyl-L-arginine-p-nitroanilide (BAPNA), was purified to near homogeneity, as judged by gel electrophoresis. The molecular weight of the enzyme was estimated to be ca. 69,000 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and ca. 50,000 by gel filtration on Sephadex G-100. The pH optimum for the hydrolysis of BAPNA was around 8.5. The enzyme was heat labile and irreversibly inactivated at low pH values. Enzyme activity was enhanced by Ca2+, Mg2+, and Ba2+ but inhibited by Mn2+, Hg2+, Co2+, and Zn2+. Metal chelators and sulfhydryl reagents had no effect on this activity. The enzyme was inhibited by certain protease inhibitors such as diisopropyl fluorophosphate, N-alpha-p-tosyl-L-lysine chloromethyl ketone, phenylmethylsulfonyl fluoride, L-1-tosylamide-2-phenylethylchloromethyl ketone, alpha-1-antitrypsin, and soybean trypsin inhibitor. The Km values for BAPNA and N-alpha-benzoyl-L-arginine ethyl ester were 0.05 and 0.12 mM, respectively, and the Vmax values were higher than those observed with trypsin. Although the purified enzyme hydrolyzed some low-molecular-weight synthetic trypsin substrates, it did not hydrolyze casein, hemoglobin, azocasein, azocoll, bovine serum albumin, or gelatin. Thus, this enzyme is probably not a protease but is capable of hydrolyzing ester, amide, and peptide bonds involving the carboxyl group of arginine and lysine.
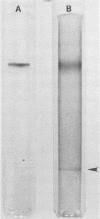
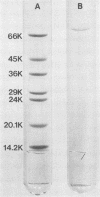
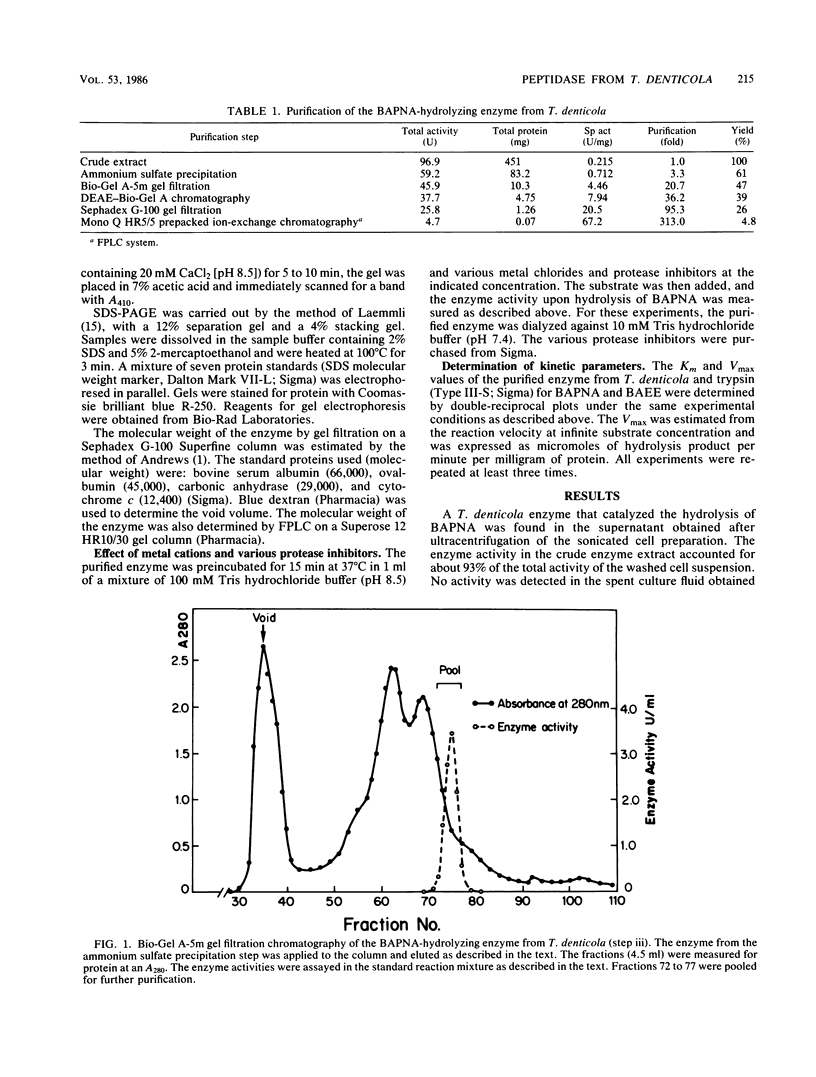
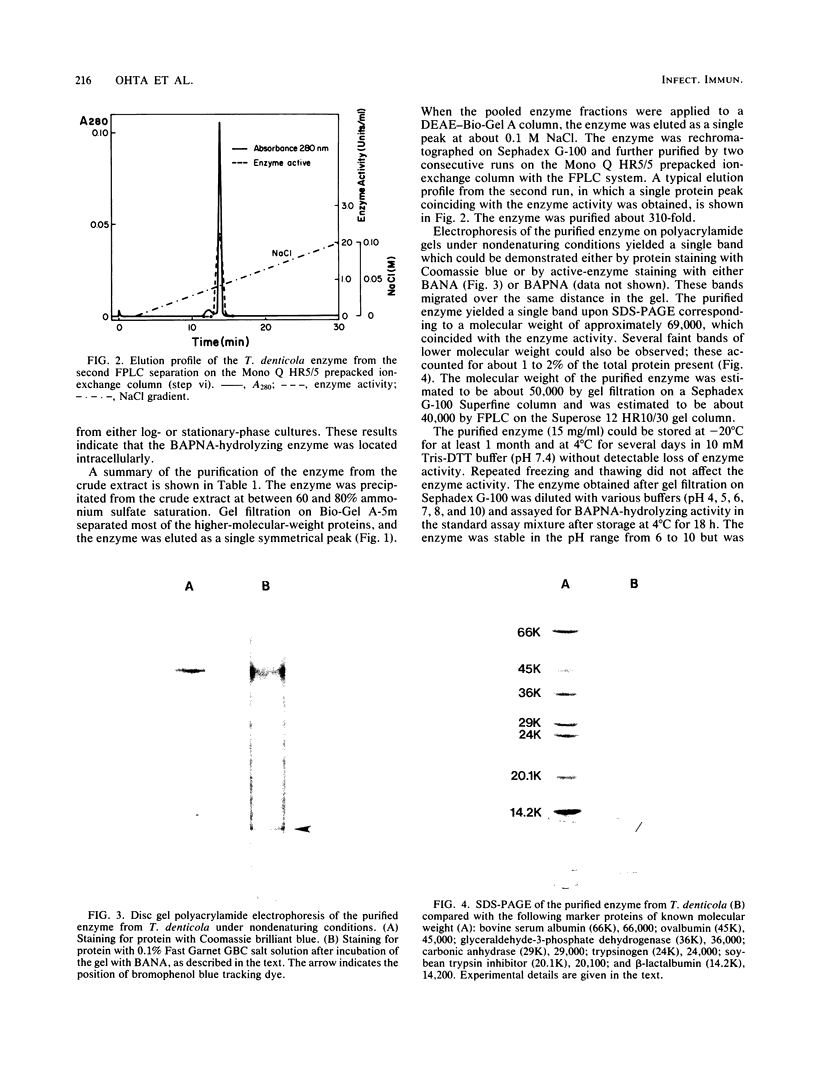
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. P., Canale-Parola E. Arginine catabolism by Treponema denticola. J Bacteriol. 1976 Nov;128(2):616–622. doi: 10.1128/jb.128.2.616-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehringer H., Taichman N. S., Shenker B. J. Suppression of fibroblast proliferation by oral spirochetes. Infect Immun. 1984 Jul;45(1):155–159. doi: 10.1128/iai.45.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Herrmann B. F., Höfling J. F., Sundqvist G. K. Degradation of the human proteinase inhibitors alpha-1-antitrypsin and alpha-2-macroglobulin by Bacteroides gingivalis. Infect Immun. 1984 Feb;43(2):644–648. doi: 10.1128/iai.43.2.644-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Frank R. M. Bacterial penetration in the apical pocket wall of advanced human periodontitis. J Periodontal Res. 1980 Nov;15(6):563–573. doi: 10.1111/j.1600-0765.1980.tb00315.x. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Kilian M. Degradation of immunoglobulins A2, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981 Dec;34(3):757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Choy W. N., Champe S. P., Goldberg A. L. Role and location of "protease I" from Escherichia coli. J Bacteriol. 1976 Dec;128(3):776–784. doi: 10.1128/jb.128.3.776-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTGARTEN M. A. ELECTRON MICROSCOPIC OBSERVATIONS ON THE BACTERIAL FLORA OF ACUTE NECROTIZING ULCERATIVE GINGIVITIS. J Periodontol. 1965 Jul-Aug;36:328–339. doi: 10.1902/jop.1965.36.4.328. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Dor R. H., Warren R. A., Kelln R. A. The relationship of serine protease activity to RNA polymerase modification and sporulation in Bacillus subtilis. J Mol Biol. 1973 May 5;76(1):103–122. doi: 10.1016/0022-2836(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Liljenberg B., Lindhe J. Juvenile periodontitis. Some microbiological, histopathological and clinical characteristics. J Clin Periodontol. 1980 Feb;7(1):48–61. doi: 10.1111/j.1600-051x.1980.tb01948.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978 May;5(2):115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Levin S. Positive correlation between the proportions of subgingival spirochetes and motile bacteria and susceptibility of human subjects to periodontal deterioration. J Clin Periodontol. 1981 Apr;8(2):122–138. doi: 10.1111/j.1600-051x.1981.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Paunio K. U., Woolfolk M. P., Hockett R. N. Collagenolytic activity of dental plaque associated with periodontal pathology. Infect Immun. 1974 Feb;9(2):329–336. doi: 10.1128/iai.9.2.329-336.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Laughon B. E., Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol. 1982 Apr;53(4):223–230. doi: 10.1902/jop.1982.53.4.223. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Morrison E. C., Kerry G. A., Higgins T., Stoll J. Metronidazole in periodontitis. I. Clinical and bacteriological results after 15 to 30 weeks. J Periodontol. 1984 Jun;55(6):325–335. doi: 10.1902/jop.1984.55.6.325. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Schmidt E., Morrison E. C. Bacterial profiles of subgingival plaques in periodontitis. J Periodontol. 1985 Aug;56(8):447–456. doi: 10.1902/jop.1985.56.8.447. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Cato E. P., Smibert R. M., Burmeister J. A., Palcanis K. G., Ranney R. R. Comparative bacteriology of juvenile periodontitis. Infect Immun. 1985 May;48(2):507–519. doi: 10.1128/iai.48.2.507-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen K. K., Mäkinen P. L. Purification and characterization of two human erythrocyte arylamidases preferentially hydrolysing N-terminal arginine or lysine residues. Biochem J. 1978 Dec 1;175(3):1051–1067. doi: 10.1042/bj1751051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Esterl W., Fritz H. Sperm Acrosin. Methods Enzymol. 1981;80(Pt 100):621–632. doi: 10.1016/s0076-6879(81)80049-3. [DOI] [PubMed] [Google Scholar]
- Nitzan D., Sperry J. F., Wilkins T. D. Fibrinolytic activity of oral anaerobic bacteria. Arch Oral Biol. 1978;23(6):465–470. doi: 10.1016/0003-9969(78)90078-x. [DOI] [PubMed] [Google Scholar]
- Okuda K., Naito Y., Ohta K., Fukumoto Y., Kimura Y., Ishikawa I., Kinoshita S., Takazoe I. Bacteriological study of periodontal lesions in two sisters with juvenile periodontitis and their mother. Infect Immun. 1984 Jul;45(1):118–121. doi: 10.1128/iai.45.1.118-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacaud M., Richaud C. Protease II from Escherichia coli. Purification and characterization. J Biol Chem. 1975 Oct 10;250(19):7771–7779. [PubMed] [Google Scholar]
- Pacaud M., Uriel J. Isolation and some propeties of a proteolytic enzyme from Escherichia coli (protease I). Eur J Biochem. 1971 Dec 10;23(3):435–442. doi: 10.1111/j.1432-1033.1971.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Saglie R., Newman M. G., Carranza F. A., Jr, Pattison G. L. Bacterial invasion of gingiva in advanced periodontitis in humans. J Periodontol. 1982 Apr;53(4):217–222. doi: 10.1902/jop.1982.53.4.217. [DOI] [PubMed] [Google Scholar]
- Shenker B. J., Listgarten M. A., Taichman N. S. Suppression of human lymphocyte responses by oral spirochetes: a monocyte-dependent phenomenon. J Immunol. 1984 Apr;132(4):2039–2045. [PubMed] [Google Scholar]
- Slots J., Dahlén G. Subgingival microorganisms and bacterial virulence factors in periodontitis. Scand J Dent Res. 1985 Apr;93(2):119–127. doi: 10.1111/j.1600-0722.1985.tb01319.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Tam Y. C., Chan E. C. Purification and characterization of hyaluronidase from oral Peptostreptococcus species. Infect Immun. 1985 Feb;47(2):508–513. doi: 10.1128/iai.47.2.508-513.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]