Abstract
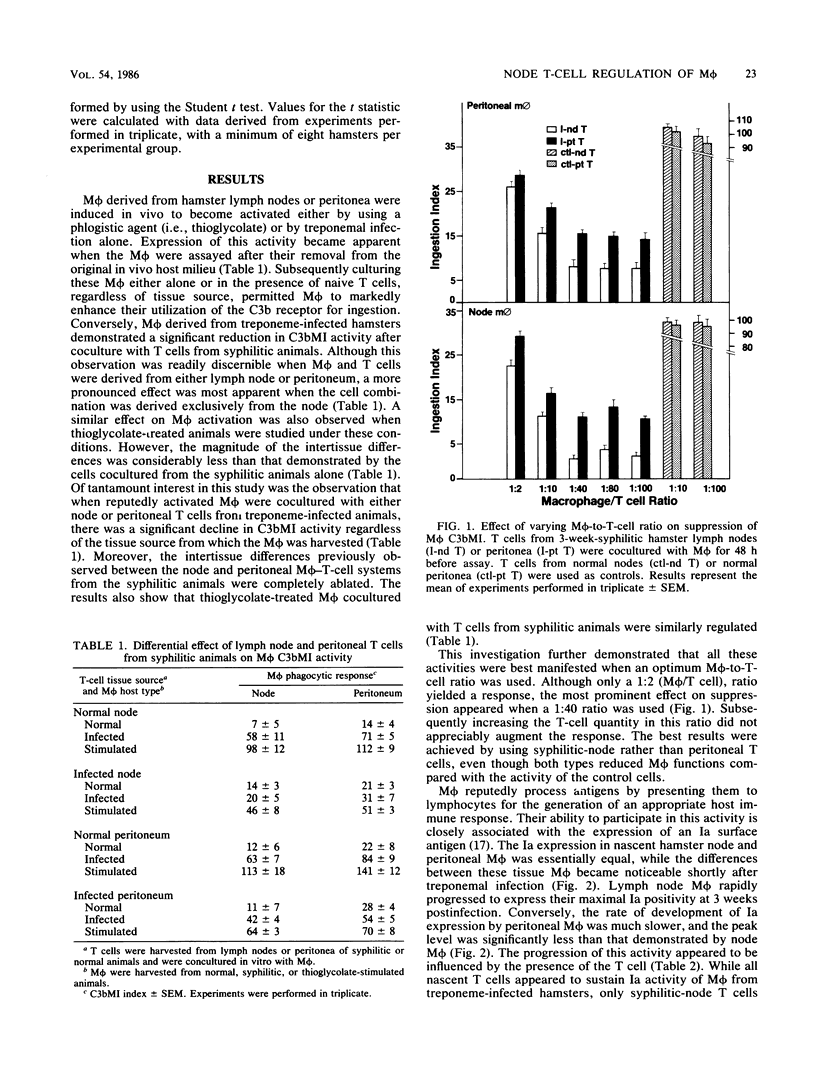
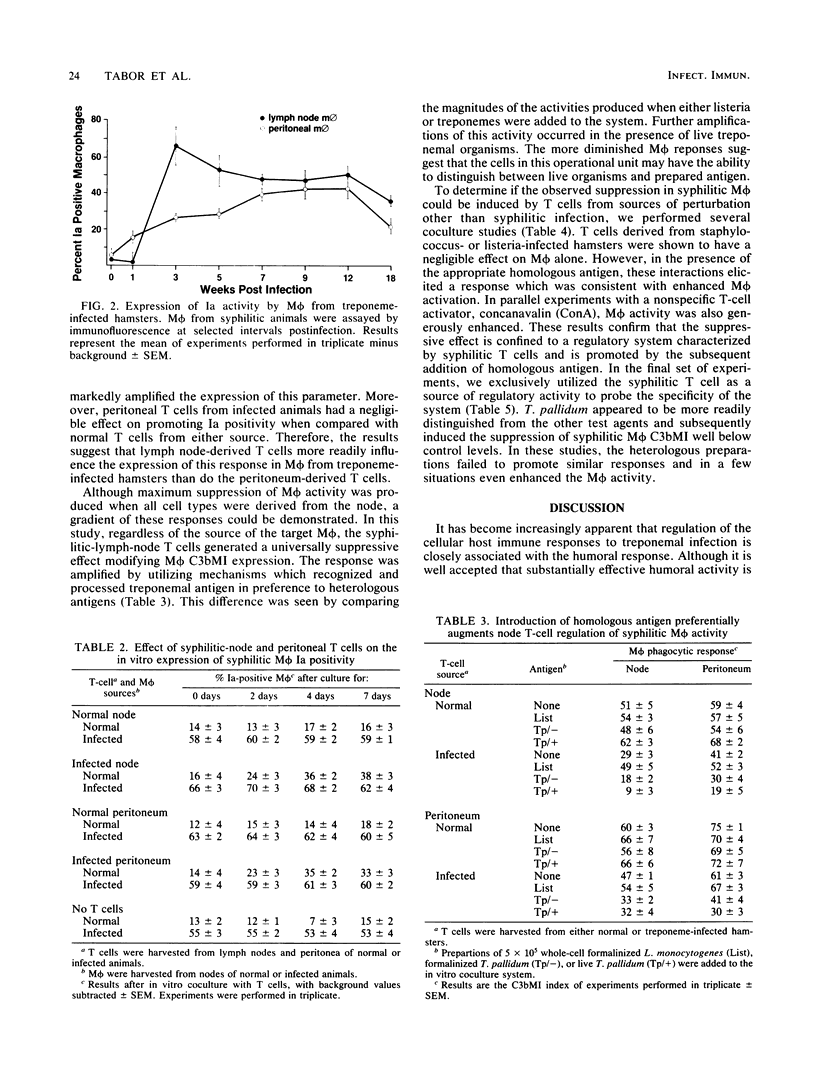
Hamsters experimentally inoculated in the inguinal region with Treponema pallidum subsp. endemicum develop considerable pathology at that site. We examined the cell populations from these inguinal lymph nodes to determine their intercellular responses to infection. In vitro, syphilitic-node T cells markedly suppressed C3b receptor-mediated ingestion (C3bMI) in syphilitic macrophages derived from sites both proximal and distal to the inoculation. This activity was more pronounced when node T cells rather than peritoneal T cells were used. When treponemal preparations or live treponemes were added to the coculture system, the suppression was specifically enhanced, whereas the addition of heterologous agents did not promote this effect. Syphilitic macrophages from either compartment cultured alone showed no significant inhibition of C3bMI. In parallel studies on syphilitic macrophages, we observed that the expression of Ia quickly became elevated and was sustained throughout the infection. Moreover, in vitro culturing of the syphilitic-node T cells with these macrophages did not alter this function. These observations suggest that the syphilitic node contains a subpopulation of T cells that can selectively suppress macrophage C3bMI activity and concurrently regulate their cellular response to treponemal infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadegan A. A., Schell R. F., LeFrock J. L. Immune serum confers protection against syphilitic infection on hamsters. Infect Immun. 1983 Oct;42(1):42–47. doi: 10.1128/iai.42.1.42-47.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. A., Chatenoud L., Wallach D., Phan Dinh Tuy F., Cottenot F. Studies on T cell subsets and functions in leprosy. Clin Exp Immunol. 1981 Jun;44(3):491–500. [PMC free article] [PubMed] [Google Scholar]
- Bagasra O., Damjanov I. Ability of macrophages to process and present Treponema pallidum Bosnia A strain antigens in experimental syphilis of syrian hamsters. Infect Immun. 1982 Apr;36(1):176–183. doi: 10.1128/iai.36.1.176-183.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O., Kushner H., Hashemi S. Lymphocyte function in experimental endemic syphilis of Syrian hamsters. Immunology. 1985 Sep;56(1):9–21. [PMC free article] [PubMed] [Google Scholar]
- Baker-Zander S. A., Hook E. W., 3rd, Bonin P., Handsfield H. H., Lukehart S. A. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis in humans. J Infect Dis. 1985 Feb;151(2):264–272. doi: 10.1093/infdis/151.2.264. [DOI] [PubMed] [Google Scholar]
- Baker-Zander S., Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Adams C. B., Musher D. M. Immune complexes in experimental syphilis: a methodologic evaluation. Sex Transm Dis. 1982 Oct-Dec;9(4):170–177. doi: 10.1097/00007435-198210000-00002. [DOI] [PubMed] [Google Scholar]
- Baughn R. E., McNeely M. C., Jorizzo J. L., Musher D. M. Characterization of the antigenic determinants and host components in immune complexes from patients with secondary syphilis. J Immunol. 1986 Feb 15;136(4):1406–1414. [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M. Isolation and preliminary characterization of circulating immune complexes from rabbits with experimental syphilis. Infect Immun. 1983 Nov;42(2):579–584. doi: 10.1128/iai.42.2.579-584.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M., Knox J. M. Effect of sensitization with Propionibacterium acnes on the growth of Listeria monocytogenes and Treponema pallidum in rabbits. J Immunol. 1977 Jan;118(1):109–113. [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J Immunol. 1976 Jul;117(1):191–196. [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976 Jul;117(1):197–207. [PubMed] [Google Scholar]
- Braathen L. R., Thorsby E. Studies on human epidermal Langerhans cells. I. Allo-activating and antigen-presenting capacity. Scand J Immunol. 1980;11(4):401–408. doi: 10.1111/j.1365-3083.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- Cowing C., Schwartz B. D., Dickler H. B. Macrophage Ia antigens. I. macrophage populations differ in their expression of Ia antigens. J Immunol. 1978 Feb;120(2):378–384. [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T. Immunological aspects of leprosy--present status. Prog Allergy. 1978;25:211–242. [PubMed] [Google Scholar]
- Graves S. Rate of clearance of virulent Treponema pallidum (Nichols) from the blood stream of normal, Mycobacterium bovis BCG-treated, and immune syphilitic rabbits. Infect Immun. 1980 Jan;27(1):264–267. doi: 10.1128/iai.27.1.264-267.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Hiai H., Elder J. H., Schwartz R. S., Khiroya R. H., Thomas C. Y., Tsichlis P. N., Coffin J. M. Expression of leukemogenic recombinant viruses associated with a recessive gene in HRS/J mice. J Exp Med. 1980 Aug 1;152(2):249–264. doi: 10.1084/jem.152.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLANDER D. H., TURNER T. B. The role of temperature in experimental treponemal infection. Am J Syph Gonorrhea Vener Dis. 1954 Nov;38(6):489–505. [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Jorizzo J. L., McNeely M. C., Baughn R. E., Solomon A. R., Cavallo T., Smith E. B. Role of circulating immune complexes in human secondary syphilis. J Infect Dis. 1986 Jun;153(6):1014–1022. doi: 10.1093/infdis/153.6.1014. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- Miller J. N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973 May;110(5):1206–1215. [PubMed] [Google Scholar]
- Musher D. M. Treponemal infections: progress toward a better understanding. J Infect Dis. 1986 Jun;153(6):1005–1006. doi: 10.1093/infdis/153.6.1005. [DOI] [PubMed] [Google Scholar]
- Nopajaroonsri C., Luk S. C., Simon G. T. Ultrastructure of the normal lymph node. Am J Pathol. 1971 Oct;65(1):1–24. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J. Antigens in immunity. XIV. Electron microscopic radioautographic studies of antigen capture in the lymph node medulla. J Exp Med. 1968 Feb 1;127(2):263–276. doi: 10.1084/jem.127.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Niederbuhl C. J., Saunders J. Antibody-mediated protection of guinea-pigs against infection with Treponema pallidum. Immunology. 1985 Oct;56(2):195–202. [PMC free article] [PubMed] [Google Scholar]
- Pavis C. S., Folds J. D., Baseman J. B. Cell-mediated immunity during syphilis. Br J Vener Dis. 1978 Jun;54(3):144–150. doi: 10.1136/sti.54.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepose J. S., Bishop N. H., Feigenbaum S., Miller J. N., Zeltzer P. M. The humoral immune response in rabbits infected with Treponema pallidum: Comparison of antibody levels measured by the staphylococcal protein A-IgG (SPA-TP) microassay with VDRL, FTA-Abs, and TPI antibody responses during the development of acquired resistance to challenge. Sex Transm Dis. 1980 Jul-Sep;7(3):125–129. [PubMed] [Google Scholar]
- Phillips J. T., Duncan W. R., Streilein J. W. The biochemical characterization of Syrian hamster cell-surface alloantigens. II. Immunochemical relationships between cell-surface alloantigens and class II MHC homologues. Immunogenetics. 1981 Mar 1;12(5-6):485–496. doi: 10.1007/BF01561690. [DOI] [PubMed] [Google Scholar]
- Phillips J. T., Streilein J. W., Proia D. A., Duncan W. R. Immunochemical characterization of Syrian hamster major histocompatibility complex homologues. Adv Exp Med Biol. 1981;134:69–85. doi: 10.1007/978-1-4757-0495-2_7. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Fehniger T. E., Silverblatt F. J., Miller J. N., Lovett M. A. The surface of virulent Treponema pallidum: resistance to antibody binding in the absence of complement and surface association of recombinant antigen 4D. Infect Immun. 1986 May;52(2):579–585. doi: 10.1128/iai.52.2.579-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M., Fitzgerald T. J. Detection and functional characterization of early appearing antibodies in rabbits with experimental syphilis. Can J Microbiol. 1985 Jan;31(1):62–67. doi: 10.1139/m85-013. [DOI] [PubMed] [Google Scholar]
- Schell R. F., Chan J. K., LeFrock J. L., Bagasra O. Endemic syphilis: transfer of resistance to Treponema pallidum strain Bosnia A in hamsters with a cell suspension enriched in thymus-derived cells. J Infect Dis. 1980 Jun;141(6):752–758. doi: 10.1093/infdis/141.6.752. [DOI] [PubMed] [Google Scholar]
- Sell S., Baker-Zander S. A., Lloyd R. M. T-cell hyperplasia of lymphoid tissues of rabbits infected with Treponema pallidum: evidence for a vigorous immune response. Sex Transm Dis. 1980 Apr-Jun;7(2):74–84. doi: 10.1097/00007435-198004000-00009. [DOI] [PubMed] [Google Scholar]
- Sell S., Baker-Zander S., Powell H. C. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab Invest. 1982 Apr;46(4):355–364. [PubMed] [Google Scholar]
- Sell S., Gamboa D., Baker-Zander S. A., Lukehart S. A., Miller J. N. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. J Invest Dermatol. 1980 Dec;75(6):470–475. doi: 10.1111/1523-1747.ep12524230. [DOI] [PubMed] [Google Scholar]
- Sell S., Norris S. J. The biology, pathology, and immunology of syphilis. Int Rev Exp Pathol. 1983;24:203–276. [PubMed] [Google Scholar]
- Sell S., Salman J., Norris S. J. Reinfection of chancre-immune rabbits with Treponema pallidum. I. Light and immunofluorescence studies. Am J Pathol. 1985 Feb;118(2):248–255. [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. Dendritic cells. Transplantation. 1981 Mar;31(3):151–155. [PubMed] [Google Scholar]
- Tabor D. R., Azadegan A. A., LeFrock J. L. The participation of activated peritoneal macrophages in Treponema pallidum subspecies pertenue infection in Syrian hamsters. J Leukoc Biol. 1985 Nov;38(5):625–634. doi: 10.1002/jlb.38.5.625. [DOI] [PubMed] [Google Scholar]
- Tabor D. R., Azadegan A. A., Schell R. F., Lefrock J. L. Inhibition of macrophage C3b-mediated ingestion by syphilitic hamster T cell-enriched fractions. J Immunol. 1984 Nov;133(5):2698–2705. [PubMed] [Google Scholar]
- Umetsu D. T., Katzen D., Jabara H. H., Geha R. S. Antigen presentation by human dermal fibroblasts: activation of resting T lymphocytes. J Immunol. 1986 Jan;136(2):440–445. [PubMed] [Google Scholar]
- Wong G. H., Steiner B., Graves S. Effect of syphilitic rabbit sera taken at different periods after infection on treponemal motility, treponemal attachment to mammalian cells in vitro, and treponemal infection in rabbits. Br J Vener Dis. 1983 Aug;59(4):220–224. doi: 10.1136/sti.59.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. J., Weingarten N. M., Baughn R. E., Duncan W. C. Studies on the pathogenesis of the Jarisch-Herxheimer reaction: development of an animal model and evidence against a role for classical endotoxin. J Infect Dis. 1982 Nov;146(5):606–615. doi: 10.1093/infdis/146.5.606. [DOI] [PubMed] [Google Scholar]


