Abstract
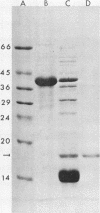
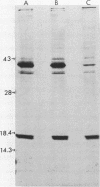
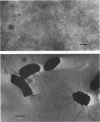
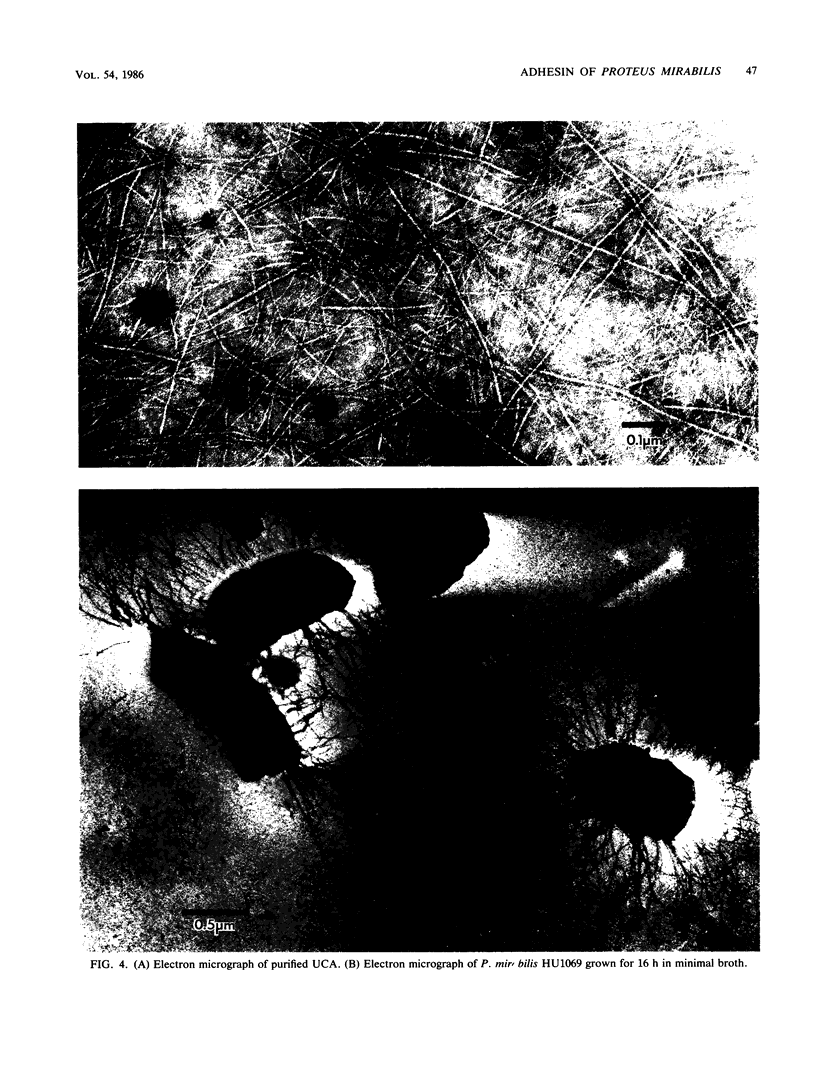
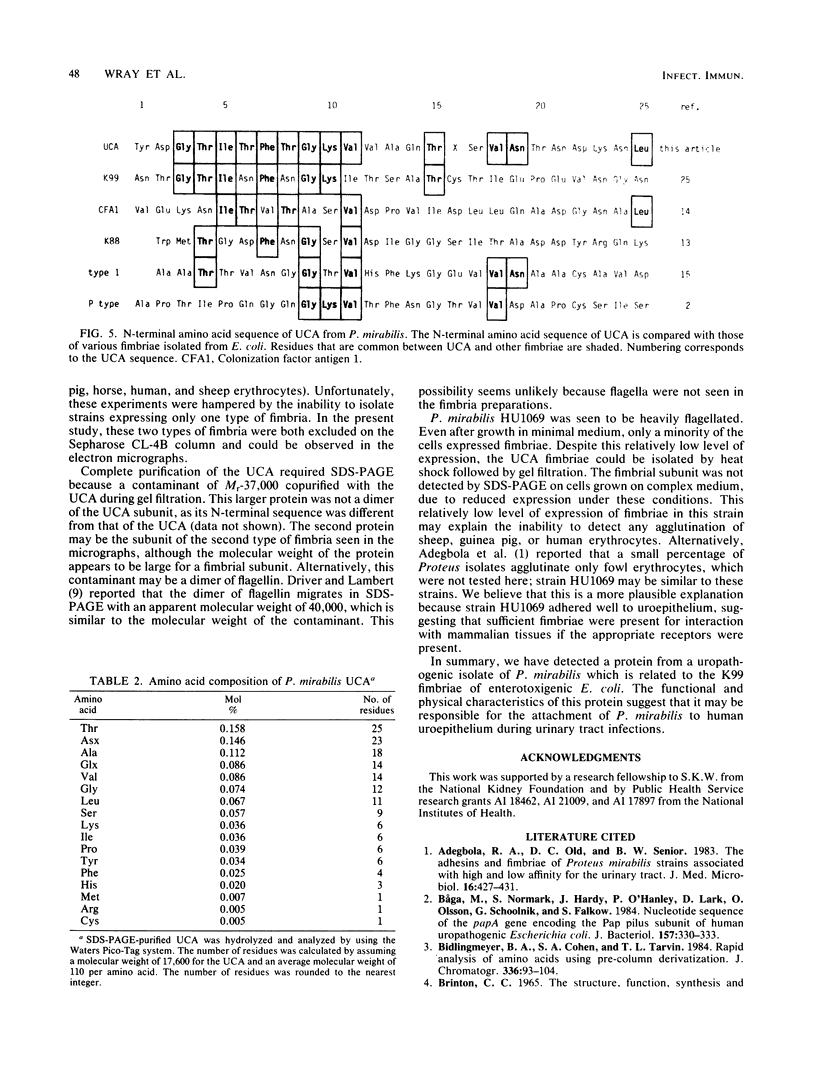
Proteus mirabilis is a frequent cause of urinary tract infections in rehabilitation hospitals and among persons with structural abnormalities of the urinary tract. Adherence to uroepithelial tissues may be an important virulence determinant in these infections because most Proteus strains adhere to desquamated uroepithelial cells. To identify the adherence factor responsible for this phenomenon, we sheared outer membrane material from 35SO4-radiolabeled bacteria and allowed it to bind to uroepithelial cells. Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the major adherence element was a protein with an apparent molecular weight of 17,500 and was provisionally designated as the uroepithelial cell adhesin. This adhesin was purified by heat shock and gel filtration on Sepharose CL-4B. After purification, the adhesin was seen assembled as long, flexible rods by electron microscopy. The N-terminal amino acid sequence of the subunit had limited homology with that of the K99 fimbriae of Escherichia coli.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adegbola R. A., Old D. C., Senior B. W. The adhesins and fimbriae of Proteus mirabilis strains associated with high and low affinity for the urinary tract. J Med Microbiol. 1983 Nov;16(4):427–431. doi: 10.1099/00222615-16-4-427. [DOI] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Bruce A. W., Chan R. C., Pinkerton D., Morales A., Chadwick P. Adherence of gram-negative uropathogens to human uroepithelial cells. J Urol. 1983 Aug;130(2):293–298. doi: 10.1016/s0022-5347(17)51115-5. [DOI] [PubMed] [Google Scholar]
- Båga M., Normark S., Hardy J., O'Hanley P., Lark D., Olsson O., Schoolnik G., Falkow S. Nucleotide sequence of the papA gene encoding the Pap pilus subunit of human uropathogenic Escherichia coli. J Bacteriol. 1984 Jan;157(1):330–333. doi: 10.1128/jb.157.1.330-333.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigen W. J., Cook R. G., Tate W. P., Caskey C. T. Bacterial peptide chain release factors: conserved primary structure and possible frameshift regulation of release factor 2. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3616–3620. doi: 10.1073/pnas.82.11.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver K., Lambert P. A. Surface antigens of Proteus mirabilis revealed by electroblotting from sodium dodecyl sulphate-polyacrylamide gels. Microbios. 1984;41(160):87–98. [PubMed] [Google Scholar]
- Edén C. S., Larsson P., Lomberg H. Attachment of Proteus mirabilis to human urinary sediment epithelial cells in vitro is different from that of Escherichia coli. Infect Immun. 1980 Mar;27(3):804–807. doi: 10.1128/iai.27.3.804-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Klemm P. Primary structure of the CFA1 fimbrial protein from human enterotoxigenic Escherichia coli strains. Eur J Biochem. 1982 May 17;124(2):339–348. doi: 10.1111/j.1432-1033.1982.tb06597.x. [DOI] [PubMed] [Google Scholar]
- Klemm P. The complete amino-acid sequence of the K88 antigen, a fimbrial protein from Escherichia coli. Eur J Biochem. 1981 Jul;117(3):617–627. doi: 10.1111/j.1432-1033.1981.tb06382.x. [DOI] [PubMed] [Google Scholar]
- Klemm P. The fimA gene encoding the type-1 fimbrial subunit of Escherichia coli. Nucleotide sequence and primary structure of the protein. Eur J Biochem. 1984 Sep 3;143(2):395–399. doi: 10.1111/j.1432-1033.1984.tb08386.x. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Nurmiaho E. L., Ranta H., Edén C. S. New Method for isolation of immunologically pure pili from Escherichia coli. Infect Immun. 1980 Feb;27(2):569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nitzan Y., Maayan M., Wajsman C., Drucker M. Urinary tract infection and drug-resistant bacteria in different patient populations. Isr J Med Sci. 1983 Dec;19(12):1039–1045. [PubMed] [Google Scholar]
- Old D. C., Adegbola R. A. Haemagglutinins and fimbriae of Morganella, Proteus and Providencia. J Med Microbiol. 1982 Nov;15(4):551–564. doi: 10.1099/00222615-15-4-551. [DOI] [PubMed] [Google Scholar]
- Pazin G. J., Braude A. I. Immobilizing antibodies in urine. II. Prevention of ascending spread of Proteus mirabilis. Invest Urol. 1974 Sep;12(2):129–133. [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Investigation of the haemolytic activity of Proteus mirabilis strains. Antonie Van Leeuwenhoek. 1983 Apr;49(1):1–11. doi: 10.1007/BF00457874. [DOI] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Vero cell invasiveness of Proteus mirabilis. Infect Immun. 1984 Mar;43(3):1068–1071. doi: 10.1128/iai.43.3.1068-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge K. A., McDonald H. P., Jr Costs of treating simple nosocomial urinary tract infection. Urology. 1985 Jul;26(1 Suppl):24–26. [PubMed] [Google Scholar]
- Senior B. W. The special affinity of particular types of Proteus mirabilis for the urinary tract. J Med Microbiol. 1979 Feb;12(1):1–8. doi: 10.1099/00222615-12-1-1. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf F. K., Roorda I. Production, purification, and characterization of the fimbrial adhesive antigen F41 isolated from calf enteropathogenic Escherichia coli strain B41M. Infect Immun. 1982 May;36(2):751–758. doi: 10.1128/iai.36.2.751-758.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]