Abstract
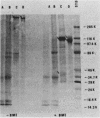
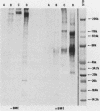
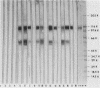
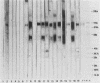
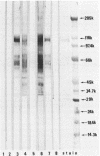
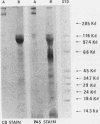
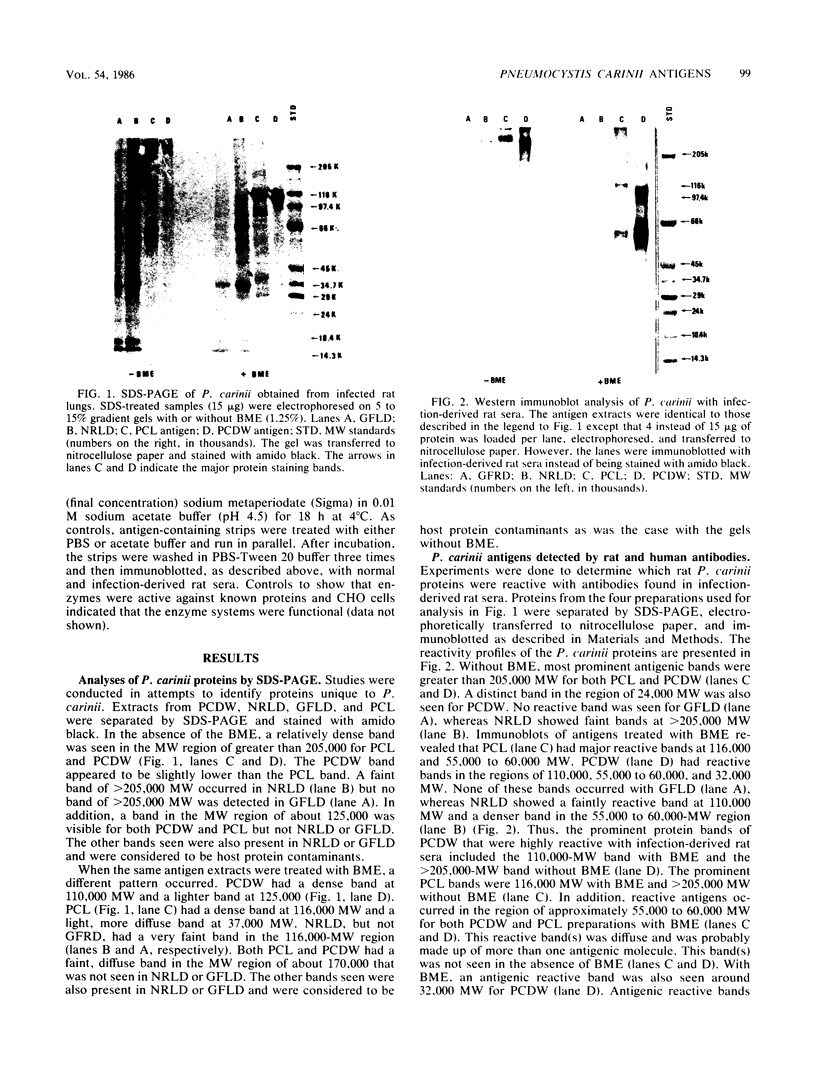
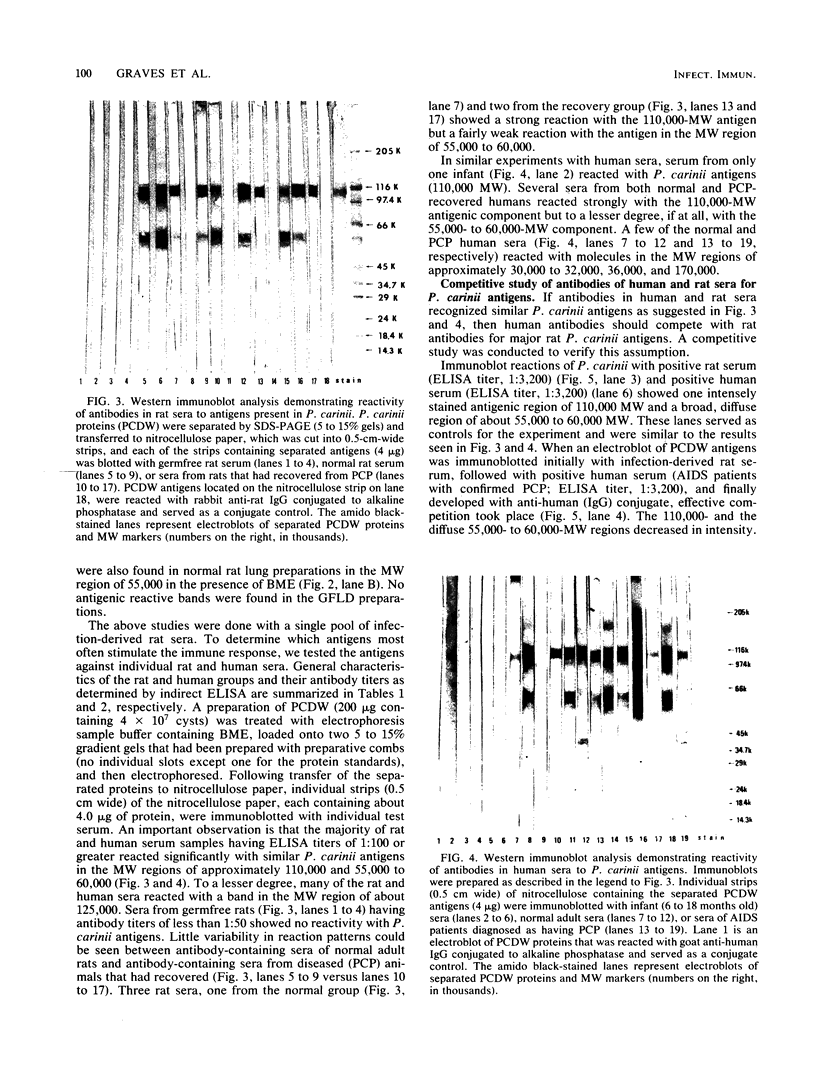
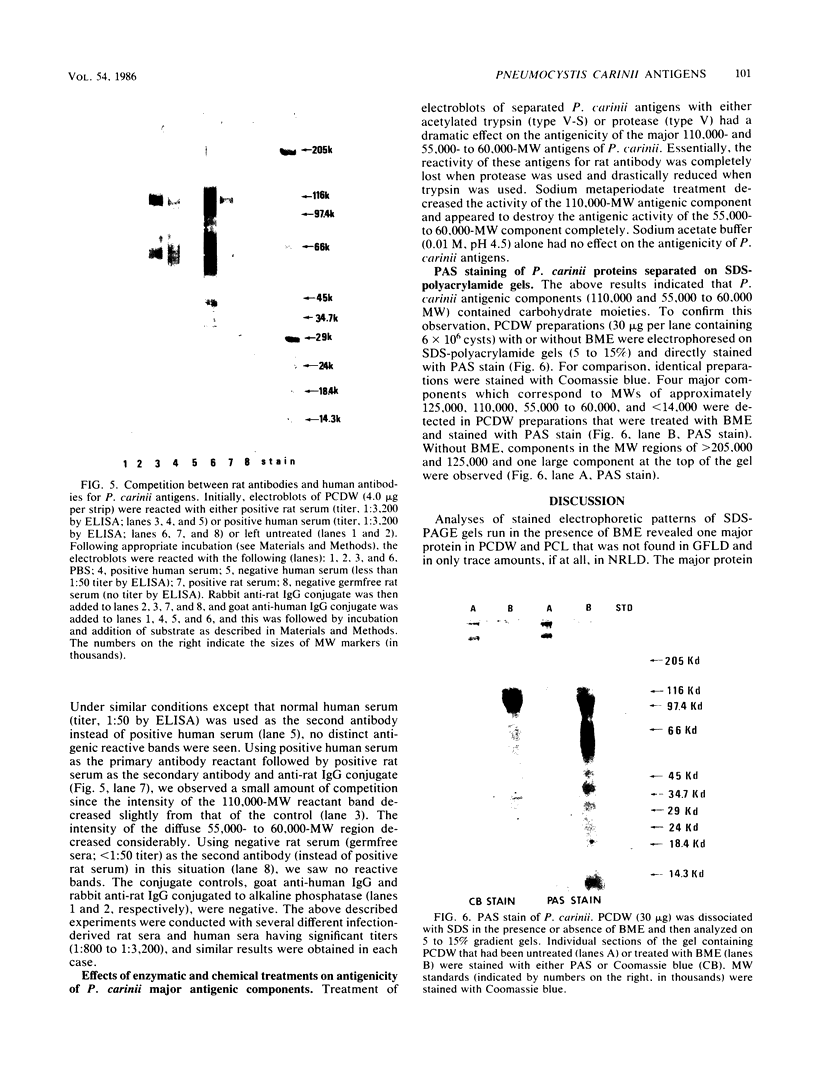
The major Pneumocystis carinii antigens inducing antibody responses in infected hosts were identified by Western immunoblotting techniques. The biochemical nature of these antigens was also elucidated. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by protein staining revealed a major component with a molecular weight (MW) of greater than 205,000. This major component disappeared and a new major protein staining component of approximately 110,000 to 116,000 MW appeared when electrophoresis was done in the presence of beta-mercaptoethanol. Periodic acid-Schiff staining revealed that this major component contains carbohydrate moieties. A major component in the 55,000- to 60,000-MW region was visible with periodic acid-Schiff stain, but not with a protein stain, after electrophoresis in the presence of beta-mercaptoethanol. The majority of sera tested from humans with diagnosed pneumocystosis and from rats allowed to recover from steroid-induced pneumocystosis reacted strongly with 110,000- to 116,000-, and 55,000- to 60,000-MW components. These sera often, but not always, detected antigens with MWs of approximately 170,000, 125,000, and 30,000 to 32,000. The data suggest that the antigenic composition of P. carinii is relatively complex and that rat and human P. carinii probably share antigenic determinants. Competitive studies between infection-derived human and rat antisera for the major rat P. carinii components revealed competition; rat antisera appeared to recognize a greater range of antigenic epitopes than did human antisera. Protease treatment of the antigenic components that had been immobilized on nitrocellulose paper destroyed their antigenic reactivity with rat antibody. Treatment with sodium periodate decreased reactivity of this 110,000- to 116,000-MW component and completely destroyed the reactivity of the 55,000- to 60,000-MW component with rat antibody.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRZOSKO W. J., NOWOSLAWSKI A. IDENTIFICATION OF PNEUMOCYSTIS CARINII ANTIGENS IN TISSUES. Bull Acad Pol Sci Biol. 1965;13:49–54. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DYER J. R. Use of periodate oxidations in biochemical analysis. Methods Biochem Anal. 1956;3:111–152. doi: 10.1002/9780470110195.ch5. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Graves D. C., McNabb S. J., Ivey M. H., Worley M. A. Development and characterization of monoclonal antibodies to Pneumocystis carinii. Infect Immun. 1986 Jan;51(1):125–133. doi: 10.1128/iai.51.1.125-133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maddison S. E., Hayes G. V., Ivey M. H., Tsang V. C., Slemenda S. B., Norman L. G. Fractionation of Pneumocystis carinii antigens used in an enzyme-linked immunosorbent assay for antibodies and in the production of antiserum for detecting Pneumocystis carinii antigenemia. J Clin Microbiol. 1982 Jun;15(6):1029–1035. doi: 10.1128/jcm.15.6.1029-1035.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison S. E., Walls K. W., Haverkos H. W., Juranek D. D. Evaluation of serologic tests for Pneumocystis carinii antibody and antigenemia in patients with acquired immunodeficiency syndrome. Diagn Microbiol Infect Dis. 1984 Jan;2(1):69–73. doi: 10.1016/0732-8893(84)90025-7. [DOI] [PubMed] [Google Scholar]
- Masur H., Jones T. C. The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J Exp Med. 1978 Jan 1;147(1):157–170. doi: 10.1084/jem.147.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OPFERKUCH W. [On the histochemistry of Pneumocystis carinii]. Virchows Arch Pathol Anat Physiol Klin Med. 1959;332:364–373. [PubMed] [Google Scholar]
- Pifer L. L., Hughes W. T., Stagno S., Woods D. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children. Pediatrics. 1978 Jan;61(1):35–41. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D. Pneumocystis carinii infection. South Med J. 1977 Nov;70(11):1330–1337. doi: 10.1097/00007611-197711000-00027. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K., Rutledge M. E., Milder J. E. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980 Mar;27(3):928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E. Comparison of rat, mouse, and human Pneumocystis carinii by immunofluorescence. J Infect Dis. 1980 Sep;142(3):449–449. doi: 10.1093/infdis/142.3.449. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E., Yoneda K., Stahr B. J. Pneumocystis carinii: new separation method from lung tissue. Exp Parasitol. 1979 Jun;47(3):356–368. doi: 10.1016/0014-4894(79)90088-2. [DOI] [PubMed] [Google Scholar]