Abstract
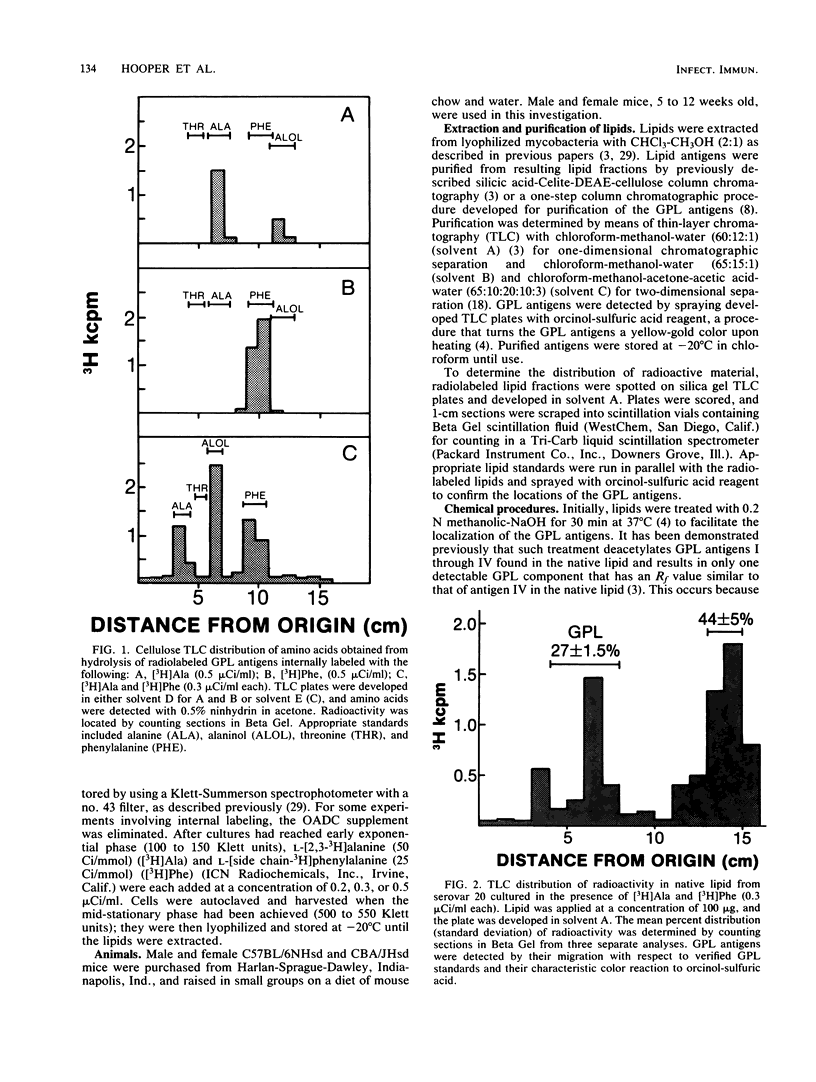
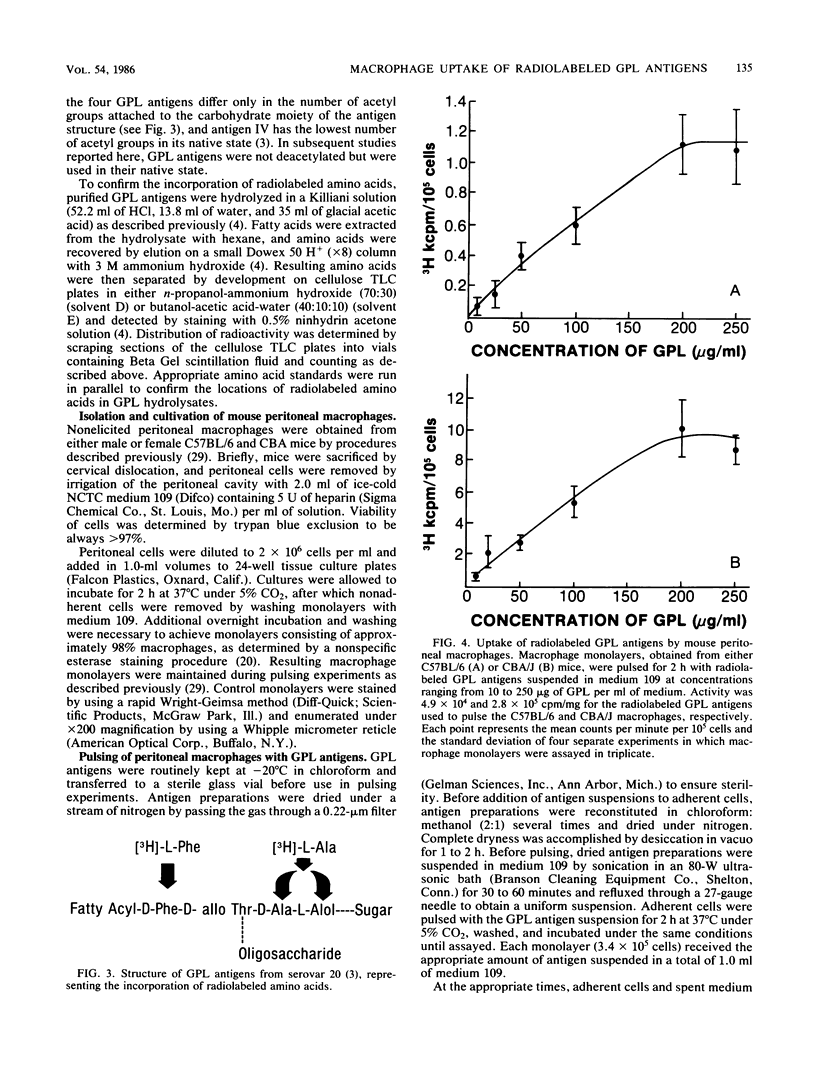
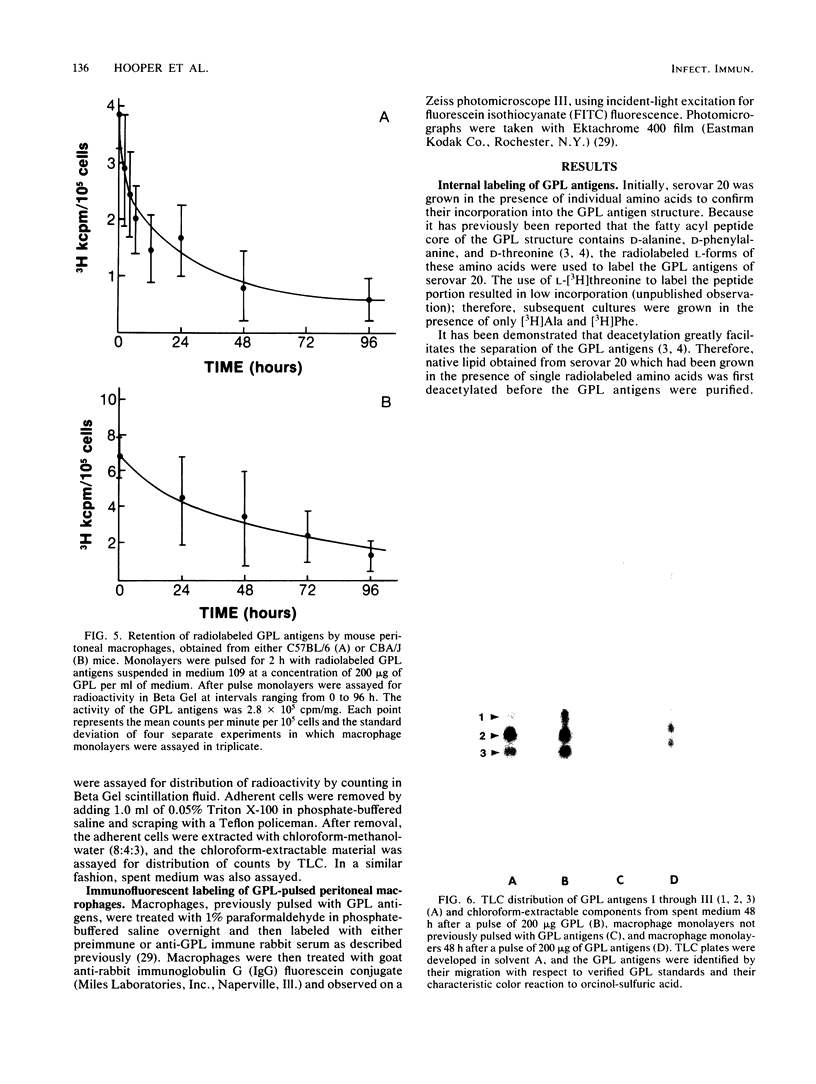
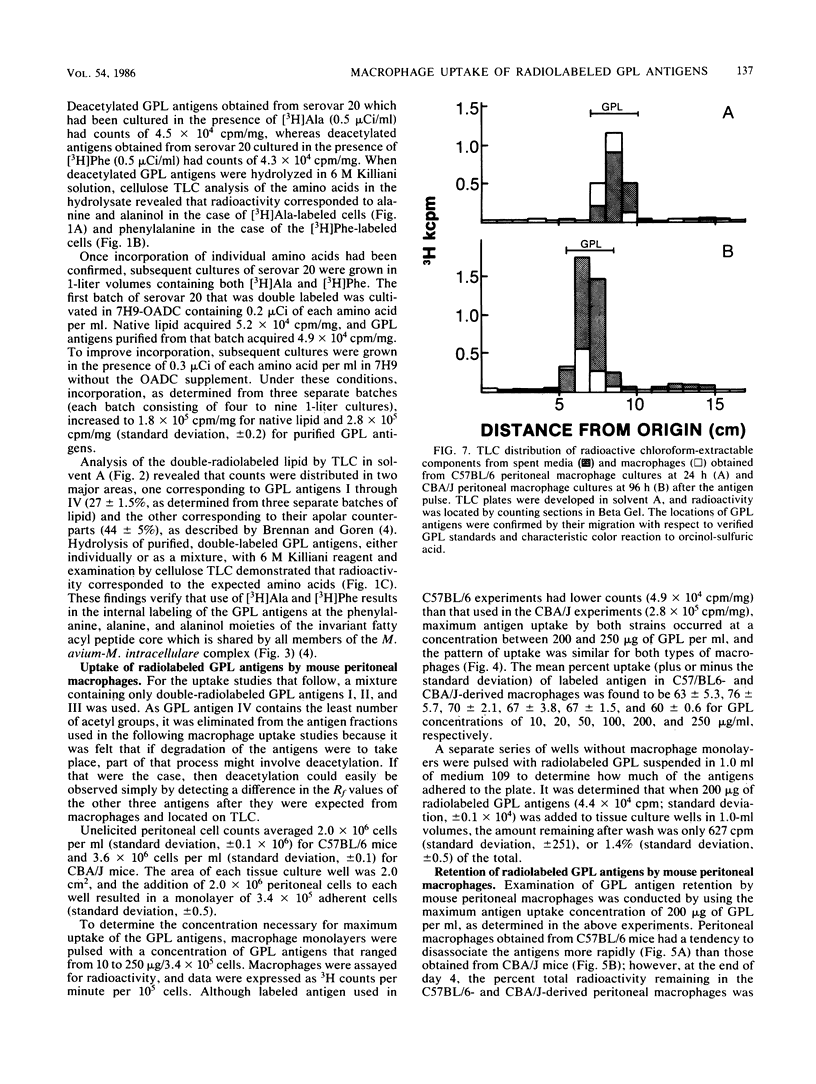
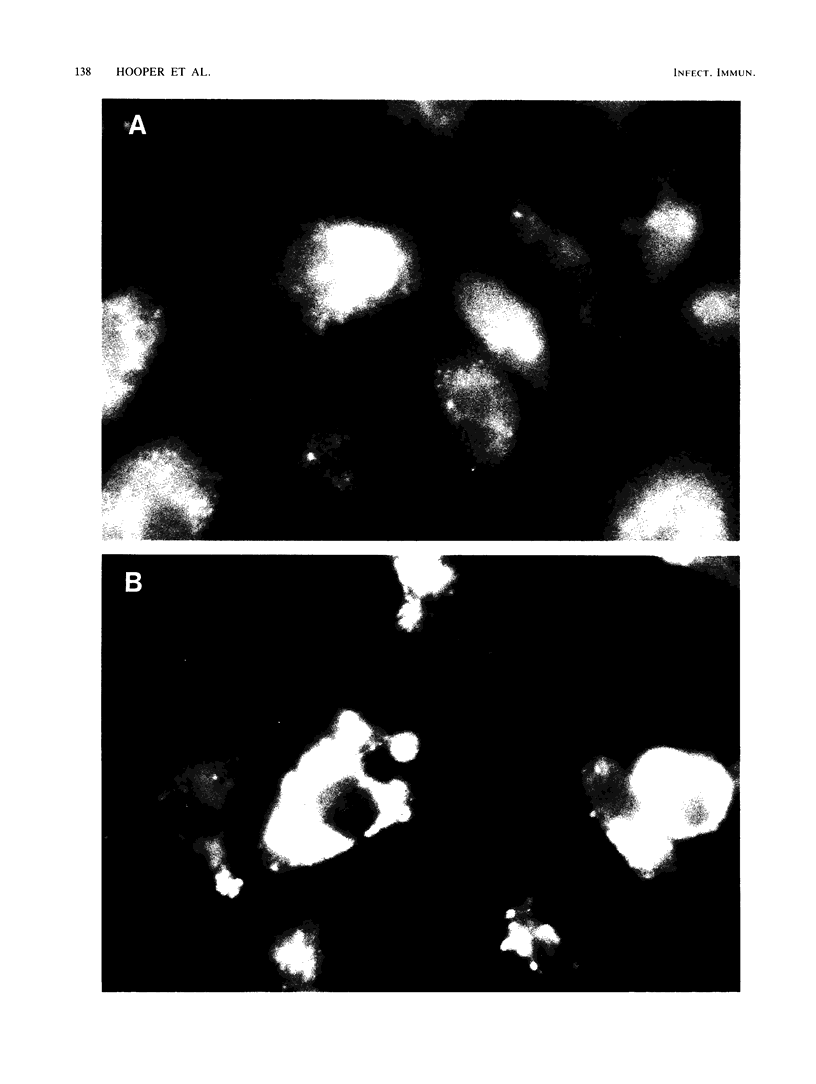
Glycopeptidolipid (GPL) antigens which are associated with the superficial L1 layer of Mycobacterium intracellulare serovar 20 were labeled with radioisotopes by means of internal labeling techniques and used in macrophage uptake and retention studies. The use of tritiated alanine and phenylalanine allowed the incorporation of label into the GPL invariant fatty acyl peptide core, which is common to all members of the Mycobacterium avium-M. intracellulare complex. Radiolabeled GPL antigens were then purified by a one-step column chromatographic procedure and subsequently used to determine the maximum uptake and retention in peritoneal macrophages isolated from C57BL/6 and CBA/J mice. Maximum uptake for peritoneal macrophages from both strains of mice occurred at a concentration between 200 and 250 micrograms of antigen per ml of medium when 3.4 X 10(5) cells were pulsed. Timed experiments demonstrated that approximately 20% of the antigens remained associated with the macrophages up to 4 days after a pulse of 200 micrograms of GPL, and examination of chloroform-extractable components from both macrophages and spent medium revealed that 98% or more of the radioactivity corresponded to intact GPL components. The ability of the GPL antigens to become associated with macrophages is demonstrated by these results, which strongly suggest that these potentially important mycobacterial antigens are inert to degradation by those cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L., Kim K. S. Mycobacterium. Bacteriol Rev. 1977 Mar;41(1):217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Brennan P. J. Immunogenicity of type-specific C-mycoside glycopeptidolipids of mycobacteria. Infect Immun. 1982 May;36(2):678–684. doi: 10.1128/iai.36.2.678-684.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Burmester G. R., Gramatzki M., Müller-Hermelink H. K., Solbach W., Djawari D., Kalden J. R. Dissociation of helper/inducer T-cell functions: immunodeficiency associated with mycobacterial histiocytosis. Clin Immunol Immunopathol. 1984 Feb;30(2):279–289. doi: 10.1016/0090-1229(84)90062-x. [DOI] [PubMed] [Google Scholar]
- Cole G. W., Gebhard J. Mycobacterium avium infection of the skin resembling lepromatous leprosy. Br J Dermatol. 1979 Jul;101(1):71–74. doi: 10.1111/j.1365-2133.1979.tb15295.x. [DOI] [PubMed] [Google Scholar]
- Davidson P. T., Khanijo V., Goble M., Moulding T. S. Treatment of disease due to Mycobacterium intracellulare. Rev Infect Dis. 1981 Sep-Oct;3(5):1052–1059. doi: 10.1093/clinids/3.5.1052. [DOI] [PubMed] [Google Scholar]
- Dimitrijevich S. D., Johnson M. M., Barrow W. W. One-step column chromatographic procedure for purification of mycobacterial glycopeptidolipid antigens. J Chromatogr. 1986 Apr 25;377:345–349. doi: 10.1016/s0378-4347(00)80791-4. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. Electron-transparent zone of mycobacteria may be a defence mechanism. Nature. 1970 Nov 28;228(5274):860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Draper P. The mycoside capsule of Mycobacterium Avium 357. J Gen Microbiol. 1974 Aug;83(2):431–433. doi: 10.1099/00221287-83-2-431. [DOI] [PubMed] [Google Scholar]
- Elliott J. L., Hoppes W. L., Platt M. S., Thomas J. G., Patel I. P., Gansar A. The acquired immunodeficiency syndrome and Mycobacterium avium-intracellulare bacteremia in a patient with hemophilia. Ann Intern Med. 1983 Mar;98(3):290–293. doi: 10.7326/0003-4819-98-3-290. [DOI] [PubMed] [Google Scholar]
- Greene J. B., Sidhu G. S., Lewin S., Levine J. F., Masur H., Simberkoff M. S., Nicholas P., Good R. C., Zolla-Pazner S. B., Pollock A. A. Mycobacterium avium-intracellulare: a cause of disseminated life-threatening infection in homosexuals and drug abusers. Ann Intern Med. 1982 Oct;97(4):539–546. doi: 10.7326/0003-4819-97-4-539. [DOI] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Cellular mechanisms of genetically controlled host resistance to Mycobacterium bovis (BCG). J Immunol. 1983 Oct;131(4):1966–1972. [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981 Dec;127(6):2417–2421. [PubMed] [Google Scholar]
- Imaeda T. Electron microscopy of leprosy lesions. Dermatol Int. 1968 Apr-Jun;7(2):116–118. doi: 10.1111/j.1365-4362.1968.tb05647.x. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Salton M. R., Barksdale L. Ultrastructure of superficial mycosidic integuments of Mycobacterium sp. J Bacteriol. 1976 Feb;125(2):739–743. doi: 10.1128/jb.125.2.739-743.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M. Identification and composition of turnip root lipids. Lipids. 1967 May;2(3):244–250. doi: 10.1007/BF02532563. [DOI] [PubMed] [Google Scholar]
- Lepoivre M., Tenu J. P., Lemaire G., Petit J. F. Antitumor activity and hydrogen peroxide release by macrophages elicited by trehalose diesters. J Immunol. 1982 Aug;129(2):860–866. [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Lincoln E. M., Gilbert L. A. Disease in children due to mycobacteria other than Mycobacterium tuberculosis. Am Rev Respir Dis. 1972 May;105(5):683–714. doi: 10.1164/arrd.1972.105.5.683. [DOI] [PubMed] [Google Scholar]
- Martin A., Bruneteau M., Michel G. Incorporation des acides aminés dans le mycoside C2 de Mycobacterium avium. Bull Soc Chim Biol (Paris) 1969 Dec 2;51(7):1231–1232. [PubMed] [Google Scholar]
- Miranda D., Vuletin J. C., Kauffman S. L. Disseminated histiocytosis and intestinal malakoplakia. Occurrence due to Mycobacterium intracellulare infection. Arch Pathol Lab Med. 1979 Jun;103(6):302–305. [PubMed] [Google Scholar]
- Mufarrij A. A., Greco M. A., Antopol S. C., Borkowsky W. The histopathology of cervical lymphadenitis caused by Mycobacterium avium-Mycobacterium intracellulare complex in an immunocompromised host. Hum Pathol. 1982 Jan;13(1):78–81. doi: 10.1016/s0046-8177(82)80142-1. [DOI] [PubMed] [Google Scholar]
- Runyon E. H. Whence mycobacteria and mycobacterioses? Ann Intern Med. 1971 Sep;75(3):467–468. doi: 10.7326/0003-4819-75-3-467. [DOI] [PubMed] [Google Scholar]
- Sohn C. C., Schroff R. W., Kliewer K. E., Lebel D. M., Fligiel S. Disseminated Mycobacterium avium-intracellulare infection in homosexual men with acquired cell-mediated immunodeficiency: a histologic and immunologic study of two cases. Am J Clin Pathol. 1983 Feb;79(2):247–252. doi: 10.1093/ajcp/79.2.247. [DOI] [PubMed] [Google Scholar]
- Tereletsky M. J., Barrow W. W. Postphagocytic detection of glycopeptidolipids associated with the superficial L1 layer of Mycobacterium intracellulare. Infect Immun. 1983 Sep;41(3):1312–1321. doi: 10.1128/iai.41.3.1312-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Mendelsohn D., Rabson A. R. Characterization of a suppressor cell-activating factor (SCAF) released by adherent cells treated with M. tuberculosis. J Immunol. 1983 May;130(5):2266–2270. [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. The specificity of suppressor T cells induced by chronic Mycobacterium avium infection in mice. Clin Exp Immunol. 1981 Jan;43(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- Yeager H., Jr, Raleigh J. W. Pulmonary disease due to Mycobacterium intracellulare. Am Rev Respir Dis. 1973 Sep;108(3):547–552. doi: 10.1164/arrd.1973.108.3.547. [DOI] [PubMed] [Google Scholar]