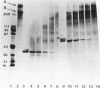
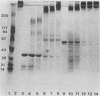
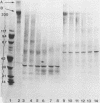
Abstract
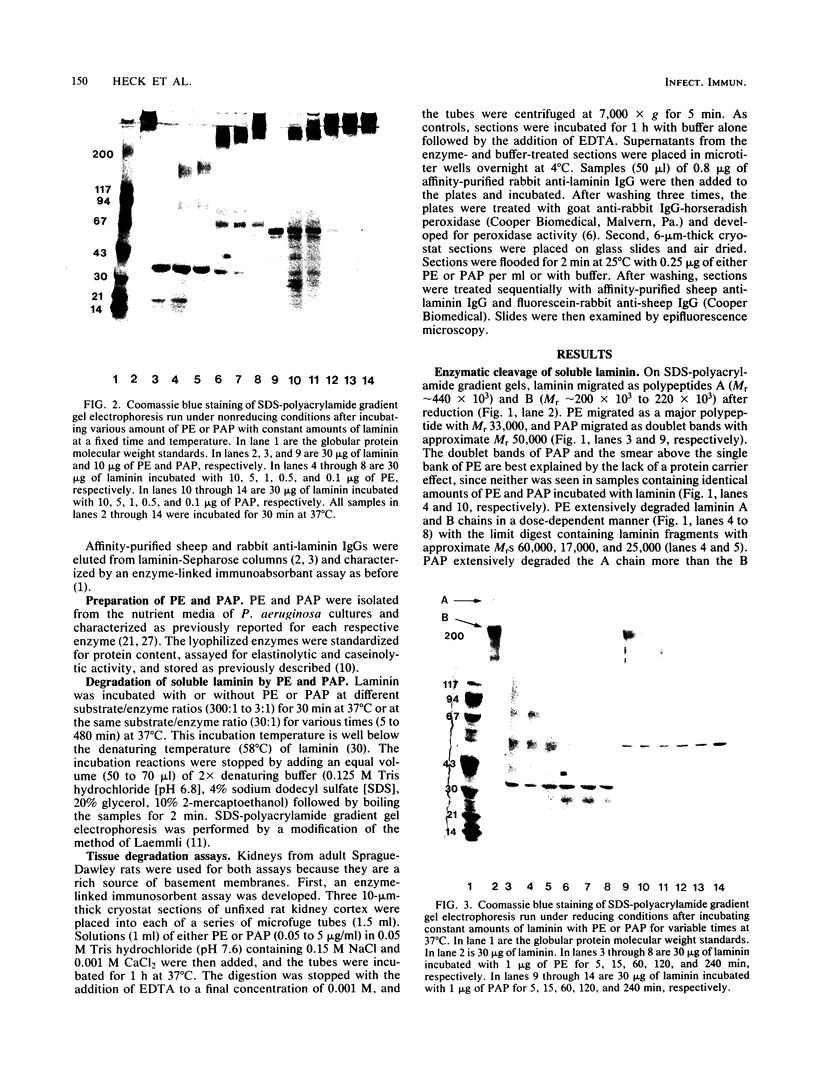
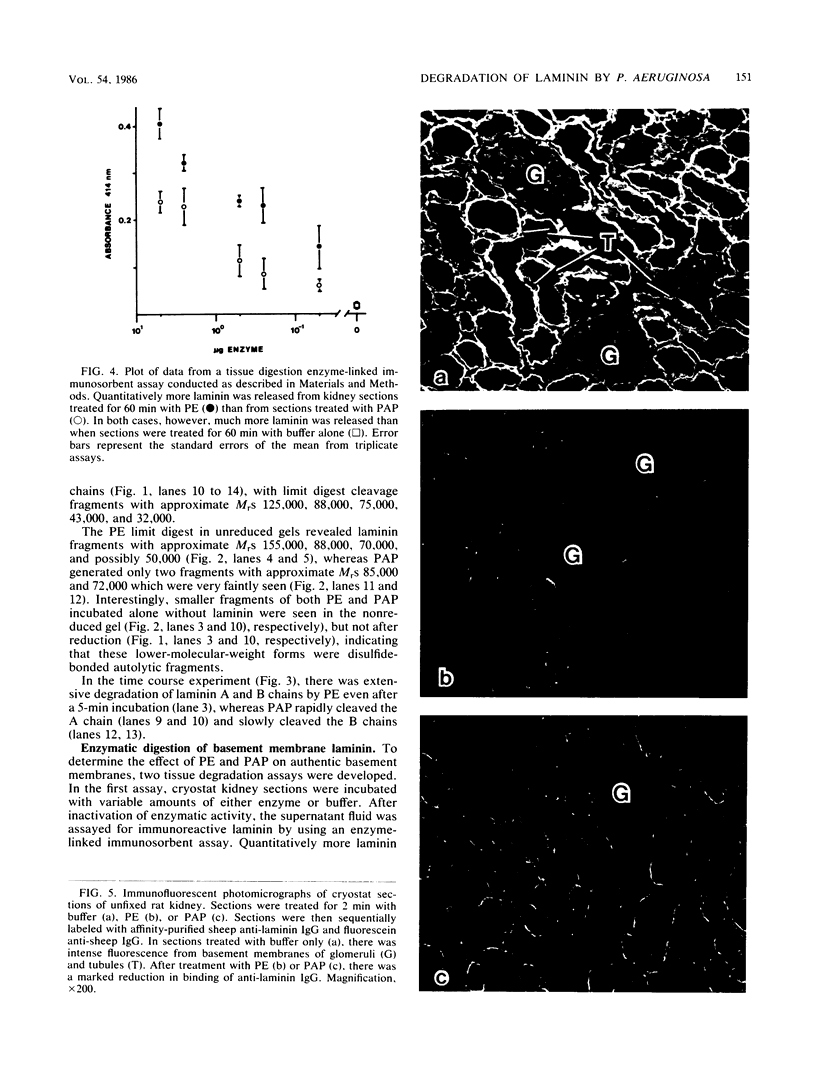
Purified Pseudomonas aeruginosa elastase and alkaline protease rapidly cleaved soluble laminin, with each enzyme yielding different cleavage products. These cleavage fragments were separated from the intact laminin A and B polypeptide chains by sodium dodecyl sulfate-polyacrylamide gradient gel electrophoresis and detected by their characteristic Coomassie blue staining patterns. Pseudomonas elastase produced rapid and extensive degradation of both A and B chains, including the disulfide-rich regions. Apparently complete degradation to limit digests was obtained after 30 min with a substrate/enzyme ratio of 30:0.5. Under similar conditions, alkaline protease rapidly degraded the A chain while slowly degrading the B chain. In addition, immunoreactive laminin was released from authentic basement membranes after incubation with either enzyme as detected by an enzyme-linked immunoabsorption assay and by immunofluorescence. The results from these studies suggest a direct role for elastase and alkaline protease in both tissue invasion and hemorrhagic tissue necrosis in P. aeruginosa infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Caulfield J. P. Distribution of laminin within rat and mouse renal, splenic, intestinal, and hepatic basement membranes identified after the intravenous injection of heterologous antilaminin IgG. Lab Invest. 1985 Feb;52(2):169–181. [PubMed] [Google Scholar]
- Abrahamson D. R., Caulfield J. P. Proteinuria and structural alterations in rat glomerular basement membranes induced by intravenously injected anti-laminin immunoglobulin G. J Exp Med. 1982 Jul 1;156(1):128–145. doi: 10.1084/jem.156.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson D. R. Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol. 1985 Jun;100(6):1988–2000. doi: 10.1083/jcb.100.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood L. L., Stone R. M., Iglewski B. H., Pennington J. E. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect Immun. 1983 Jan;39(1):198–201. doi: 10.1128/iai.39.1.198-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Ceuppens J. L., Goodwin J. S. Regulation of immunoglobulin production in pokeweed mitogen-stimulated cultures of lymphocytes from young and old adults. J Immunol. 1982 Jun;128(6):2429–2434. [PubMed] [Google Scholar]
- Döring G., Dalhoff A., Vogel O., Brunner H., Dröge U., Botzenhart K. In vivo activity of proteases of Pseudomonas aeruginosa in a rat model. J Infect Dis. 1984 Apr;149(4):532–537. doi: 10.1093/infdis/149.4.532. [DOI] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K. Extrazelluläre Toxine von Pseudomonas aeruginosa. II. Einwirkung zweier gereinigter Proteasen auf die menschlichen Immunoglobuline IgG, IgA und sekretorisches IgA. Zentralbl Bakteriol A. 1981 Mar;249(1):89–98. [PubMed] [Google Scholar]
- Gray L., Kreger A. Microscopic characterization of rabbit lung damage produced by Pseudomonas aeruginosa proteases. Infect Immun. 1979 Jan;23(1):150–159. doi: 10.1128/iai.23.1.150-159.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck L. W., Morihara K., McRae W. B., Miller E. J. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect Immun. 1986 Jan;51(1):115–118. doi: 10.1128/iai.51.1.115-118.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck L. W., Remold-O'Donnell E., Remold H. G. DFP-sensitive polypeptides of the guinea pig peritoneal macrophage. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1576–1583. doi: 10.1016/0006-291x(78)91401-8. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Wheeler R. Experimental studies of the pathogenesis of infections owing to Pseudomonas aeruginosa: elastase, an IgG protease. Can J Microbiol. 1984 Sep;30(9):1118–1124. doi: 10.1139/m84-175. [DOI] [PubMed] [Google Scholar]
- Homma J. Y., Matsuura M., Shibata M., Kazuyama Y., Yamamoto M., Kubota Y., Hirayama T., Kato I. Production of leukocidin by clinical isolates of Pseudomonas aeruginosa and antileukocidin antibody from sera of patients with diffuse panbronchiolitis. J Clin Microbiol. 1984 Nov;20(5):855–859. doi: 10.1128/jcm.20.5.855-859.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot J., Tournier J. M., Puchelle E. In vitro evidence that human airway lysozyme is cleaved and inactivated by Pseudomonas aeruginosa elastase and not by human leukocyte elastase. Infect Immun. 1985 Feb;47(2):555–560. doi: 10.1128/iai.47.2.555-560.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaharajo K., Homma J. Y., Aoyama Y., Okada K., Morihara K. Effects of protease and elastase from Pseudomonas aeruginosa on skin. Jpn J Exp Med. 1975 Apr;45(2):79–88. [PubMed] [Google Scholar]
- Kreger A. S., Gray L. D. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun. 1978 Feb;19(2):630–648. doi: 10.1128/iai.19.2.630-648.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu P. V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S94–S99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H., OKA T., INOUE H., EBATA M. PSEUDOMONAS AERUGINOSA ELASTASE. ISOLATION, CRYSTALLIZATION, AND PRELIMINARY CHARACTERIZATION. J Biol Chem. 1965 Aug;240:3295–3304. [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Comparative study of various neutral proteinases from microorganisms: specificity with oligopeptides. Arch Biochem Biophys. 1971 Sep;146(1):291–296. doi: 10.1016/s0003-9861(71)80066-8. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oda K. Protease and elastase of Pseudomonas aeruginosa: inactivation of human plasma alpha 1-proteinase inhibitor. Infect Immun. 1979 Apr;24(1):188–193. doi: 10.1128/iai.24.1.188-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oka T. On the specificity of Pseudomonas aeruginosa alkaline proteinase with synthetic peptides. Biochim Biophys Acta. 1973 Jun 6;309(2):414–429. doi: 10.1016/0005-2744(73)90040-5. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Substrate specificity of elastolytic and nonelastolytic proteinases from Pseudomonas aeruginosa. Arch Biochem Biophys. 1966 Apr;114(1):158–165. doi: 10.1016/0003-9861(66)90317-1. [DOI] [PubMed] [Google Scholar]
- Moskowitz R. W., Heinrich G. Bacterial inactivation of human serum alpha-1 antitrypsin. J Lab Clin Med. 1971 May;77(5):777–785. [PubMed] [Google Scholar]
- Mull J. D., Callahan W. S. The role of the elastase of Pseudomonas aeruginosa in experimental infection. Exp Mol Pathol. 1965 Dec;4(6):567–575. doi: 10.1016/0014-4800(65)90037-7. [DOI] [PubMed] [Google Scholar]
- Ott U., Odermatt E., Engel J., Furthmayr H., Timpl R. Protease resistance and conformation of laminin. Eur J Biochem. 1982 Mar;123(1):63–72. doi: 10.1111/j.1432-1033.1982.tb06499.x. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979 Apr;24(1):181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D. R., Miller K. D. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun. 1974 Jul;10(1):128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Vercellotti G. M., McCarthy J. B., Lindholm P., Peterson P. K., Jacob H. S., Furcht L. T. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985 Jul;120(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- Woodley D. T., Rao C. N., Hassell J. R., Liotta L. A., Martin G. R., Kleinman H. K. Interactions of basement membrane components. Biochim Biophys Acta. 1983 Dec 27;761(3):278–283. doi: 10.1016/0304-4165(83)90077-6. [DOI] [PubMed] [Google Scholar]