Abstract
A 38-kilodalton (kDa) protein antigen from Mycobacterium tuberculosis was purified by monoclonal antibody TB71-based affinity chromatography. This molecule carries two nonoverlapping epitopes recognized by monoclonal antibodies TB71 and TB72, which are expressed substantially more strongly by M. tuberculosis than by Mycobacterium bovis. However, cross-reactive determinants between these two species were revealed on the 38-kDa protein by a rabbit anti-BCG serum. An immunoradiometric assay based on the TB71 and TB72 antibody pair specifically determined 38-kDa-antigen concentrations in mycobacterial extracts. Antibodies in sera from tuberculosis patients estimated by binding to 38-kDa-antigen-coated microtiter plates were positively correlated with TB72 competing titers. Unlike antibodies, T-cell proliferative responses to the 38-kDa protein were expressed equally by 60% of tuberculosis patients and healthy BCG-vaccinated subjects. Similarly, delayed-type hypersensitivity skin reactions were elicited in both M. tuberculosis- and M. bovis-sensitized guinea pigs. The results suggest the immunodominance of the species-specific B-cell and cross-reactive T-cell stimulatory epitopes.
Full text
PDF

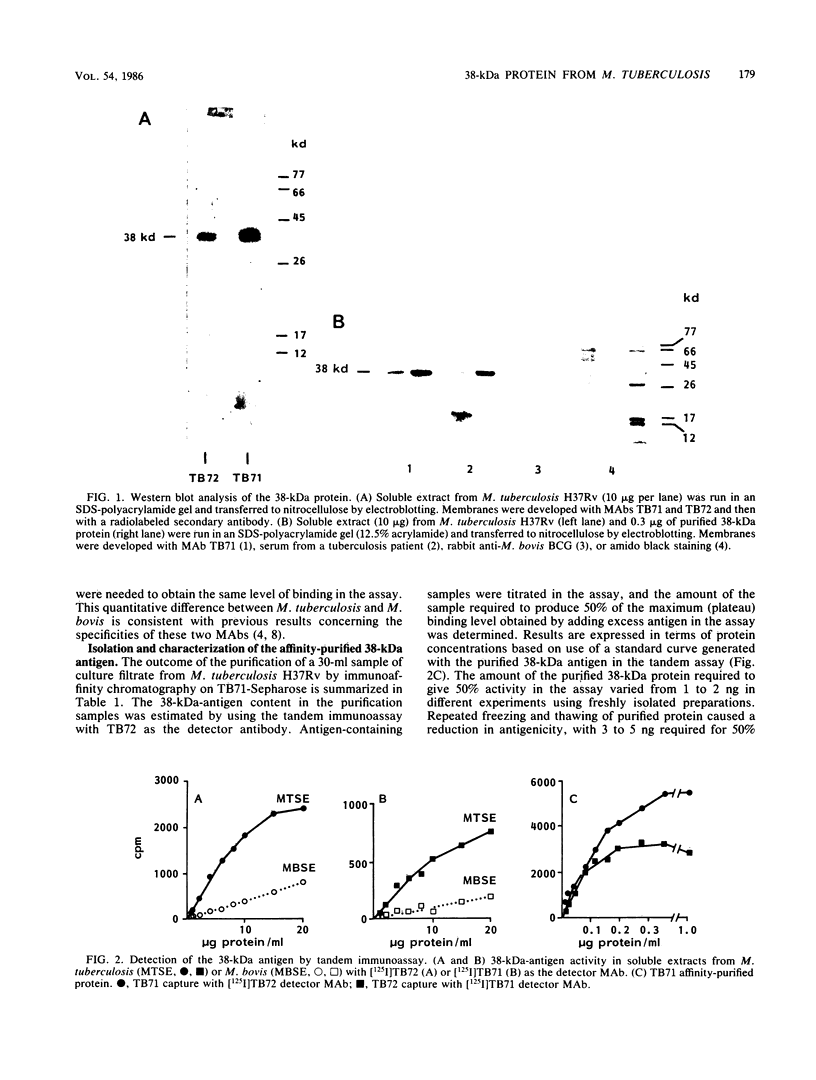

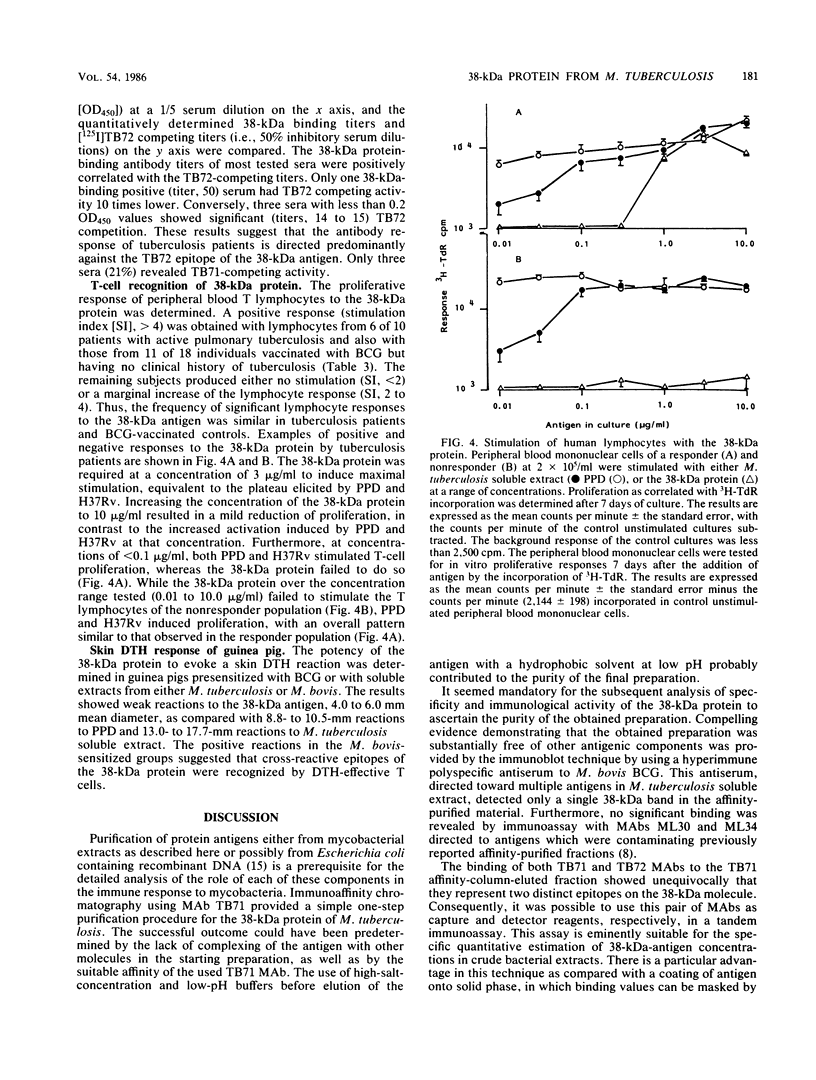


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzofsky J. A., Richman L. K., Killion D. J. Distinct H-2-linked Ir genes control both antibody and T cell responses to different determinants on the same antigen, myoglobin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4046–4050. doi: 10.1073/pnas.76.8.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürk R. R., Eschenbruch M., Leuthard P., Steck G. Sensitive detection of proteins and peptides in polyacrylamide gels after formaldehyde fixation. Methods Enzymol. 1983;91:247–254. doi: 10.1016/s0076-6879(83)91021-2. [DOI] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Balestrino E. A., Balestrino O. C., Davidson P. T., Debanne S. M., Kataria S., Kataria Y. P., Scocozza J. B. The tuberculin specificity in humans of Mycobacterium tuberculosis antigen 5. Am Rev Respir Dis. 1982 Oct;126(4):600–606. doi: 10.1164/arrd.1982.126.4.600. [DOI] [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Krambovitis E., Keen M. Evaluation of a monoclonal antibody (TB72) based serological test for tuberculosis. Clin Exp Immunol. 1983 Nov;54(2):337–345. [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S. Purification and properties of tuberculin-active protein from Mycobacterium tuberculosis. J Biol Chem. 1975 Apr 10;250(7):2556–2562. [PubMed] [Google Scholar]
- Lamb J. R., Green N. Analysis of the antigen specificity of influenza haemagglutinin-immune human T lymphocyte clones: identification of an immunodominant region for T cells. Immunology. 1983 Dec;50(4):659–666. [PMC free article] [PubMed] [Google Scholar]
- Minden P., Kelleher P. J., Freed J. H., Nielsen L. D., Brennan P. J., McPheron L., McClatchy J. K. Immunological evaluation of a component isolated from Mycobacterium bovis BCG with a monoclonal antibody to M. bovis BCG. Infect Immun. 1984 Nov;46(2):519–525. doi: 10.1128/iai.46.2.519-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyk G., Williams E. B., Nitecki D. E., Goodman J. W. The functional dissection of an antigen molecule: specificity of humoral and cellular immune responses to glucagon. J Exp Med. 1971 Jun 1;133(6):1294–1308. doi: 10.1084/jem.133.6.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Fohn M. J., Khanolkar S. R., Buchanan T. M. Monoclonal antibodies to a 28,000 mol. wt protein antigen of Mycobacterium leprae. Clin Exp Immunol. 1985 Jun;60(3):546–552. [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]



