Abstract
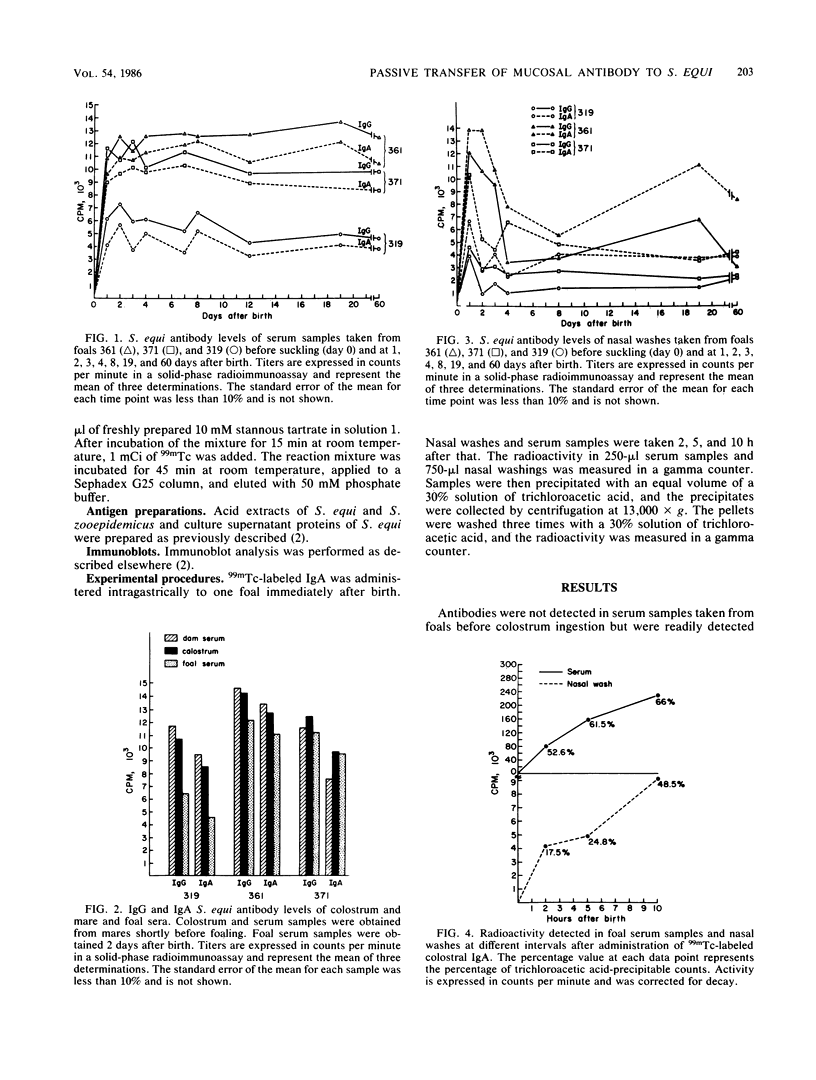
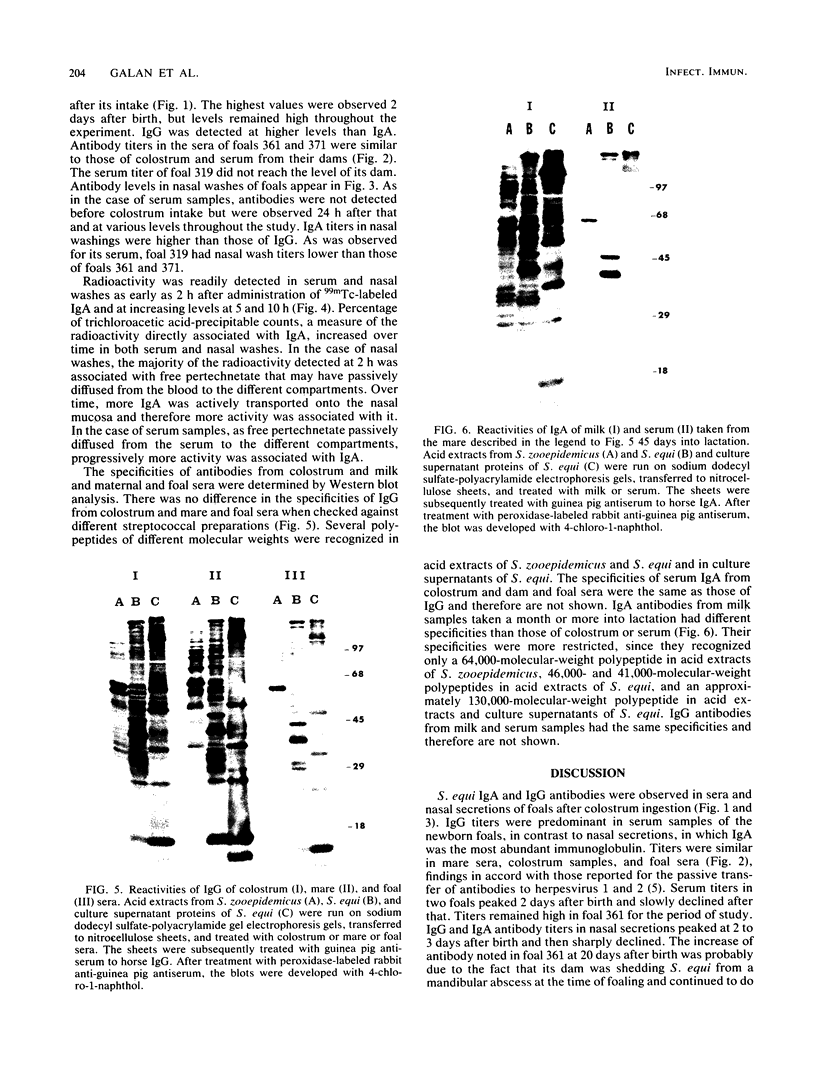
Passive transfer of mucosal antibody to Streptococcus equi was studied in foals during the first 2 months of life. Immunoglobulin G (IgG) and IgA antibodies were found in sera and nasal secretions of foals shortly after colostrum intake. Titers were highest 2 days after birth; IgG predominated in sera, and IgA predominated in nasal washes. Intragastrically administered 99mTc-labeled IgA was transported from the bloodstream to the nasal mucosa of a newborn foal within a few hours of colostrum intake. Western blot analysis of the specificities of colostral and serum antibodies showed that selective transfer of immunoglobulins of defined specificity did not occur. Antibodies from milk samples taken a month or more into lactation had different specificities than those of colostrum or serum samples. Acid-extracted M protein fragments of S. equi recognized by milk antibodies were the same as those recognized by IgG and IgA from nasopharyngeal mucus of horses recently recovered from strangles. We postulate that passive antibody protection of the foal is derived both by secretion of colostral immunoglobulins onto the nasopharyngeal mucosa and by immunoglobulins ingested in milk that directly coat the upper respiratory and oral mucosa during the first months of life.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley P. A., Bourne F. J., Brown P. J. The respiratory tract immune system in the pig. I. Distribution of immunoglobulin-containing cells in the respiratory tract mucosa. Vet Pathol. 1976;13(2):81–89. doi: 10.1177/030098587601300201. [DOI] [PubMed] [Google Scholar]
- Galan J. E., Timoney J. F. Mucosal nasopharyngeal immune responses of horses to protein antigens of Streptococcus equi. Infect Immun. 1985 Mar;47(3):623–628. doi: 10.1128/iai.47.3.623-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcott L. B. Passive immunity and its transfer with special reference to the horse. Biol Rev Camb Philos Soc. 1972 Nov;47(4):439–464. doi: 10.1111/j.1469-185x.1972.tb01078.x. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B., Hallowell A. L., Macomber L. E. Failure of colostral immunoglobulin transfer as an explanation for most infections and deaths of neonatal foals. J Am Vet Med Assoc. 1977 Jun 1;170(11):1302–1304. [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B. Identification and quantitation of equine serum and secretory immunoglobulin A. Infect Immun. 1972 Oct;6(4):610–615. doi: 10.1128/iai.6.4.610-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B. Passive immunity in the foal: measurement of immunoglobulin classes and specific antibody. Am J Vet Res. 1973 Oct;34(10):1299–1303. [PubMed] [Google Scholar]
- Morgan K. L., Bourne F. J. Immunoglobulin content of the respiratory tract secretions of piglets from birth to 10 weeks old. Res Vet Sci. 1981 Jul;31(1):40–42. [PubMed] [Google Scholar]
- Porter P. Structural and functional characteristics of immunoglobulins of the common domestic species. Adv Vet Sci Comp Med. 1979;23:1–21. doi: 10.1016/b978-0-12-039223-0.50007-x. [DOI] [PubMed] [Google Scholar]
- Reuman P. D., Paganini C. M., Ayoub E. M., Small P. A., Jr Maternal-infant transfer of influenza-specific immunity in the mouse. J Immunol. 1983 Feb;130(2):932–936. [PubMed] [Google Scholar]
- Sheldrake R. F., Husband A. J., Watson D. L., Cripps A. W. Selective transport of serum-derived IgA into mucosal secretions. J Immunol. 1984 Jan;132(1):363–368. [PubMed] [Google Scholar]
- Smith W. D., Dawson A. M., Wells P. W., Burrells C. Immunoglobulins in the serum and nasal secretions of lambs following vaccination and aerosol challenge with parainfluenza 3 virus. Res Vet Sci. 1976 Nov;21(3):341–348. [PubMed] [Google Scholar]
- Smith W. D., Wells P. W., Burrells C., Dawson A. M. Maternal immunoglobulins and parainfluenza 3 virus inhibitors in the nasal and lachrymal secretions and serum of newborn lambs. Clin Exp Immunol. 1976 Mar;23(3):544–553. [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Barnum D. A. Adherence of Streptococcus equi on tongue, cheek and nasal epithelial cells of ponies. Vet Microbiol. 1983 Oct;8(5):493–504. doi: 10.1016/0378-1135(83)90043-3. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]
- Wells P. W., Dawson A. M., Smith W. D., Smith B. S. Transfer of IgG from plasma to nasal secretions in newborn lambs. Vet Rec. 1975 Dec 6;97(23):455–455. [PubMed] [Google Scholar]
- Wilkie B. N. Respiratory tract immune response to microbial pathogens. J Am Vet Med Assoc. 1982 Nov 15;181(10):1074–1079. [PubMed] [Google Scholar]
- Yang S. S., Nickoloff E. L., McIntyre P. A., Maddrey W. C., Mikesell H. H., Scheffel U., Kasecamp W., MacAllister N. P. Tc-99m human serum albumin: a suitable agent for plasma volume measurements in man. J Nucl Med. 1978 Jul;19(7):804–807. [PubMed] [Google Scholar]




