Abstract
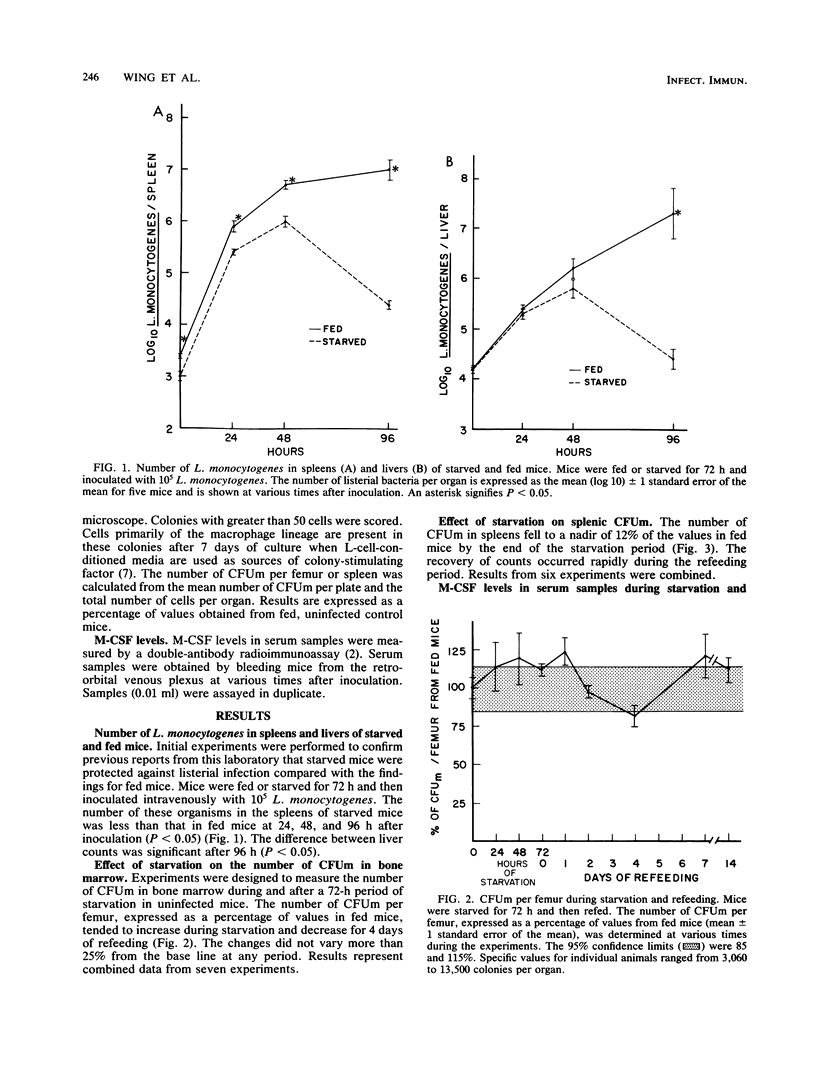
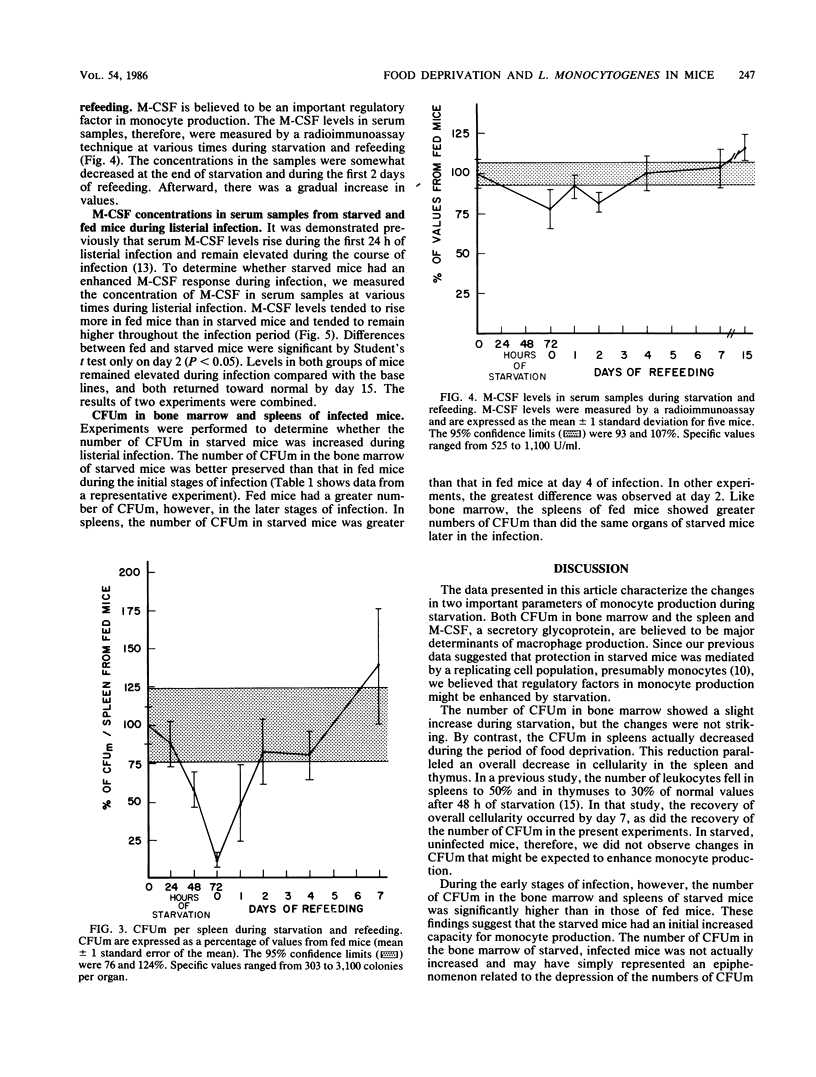
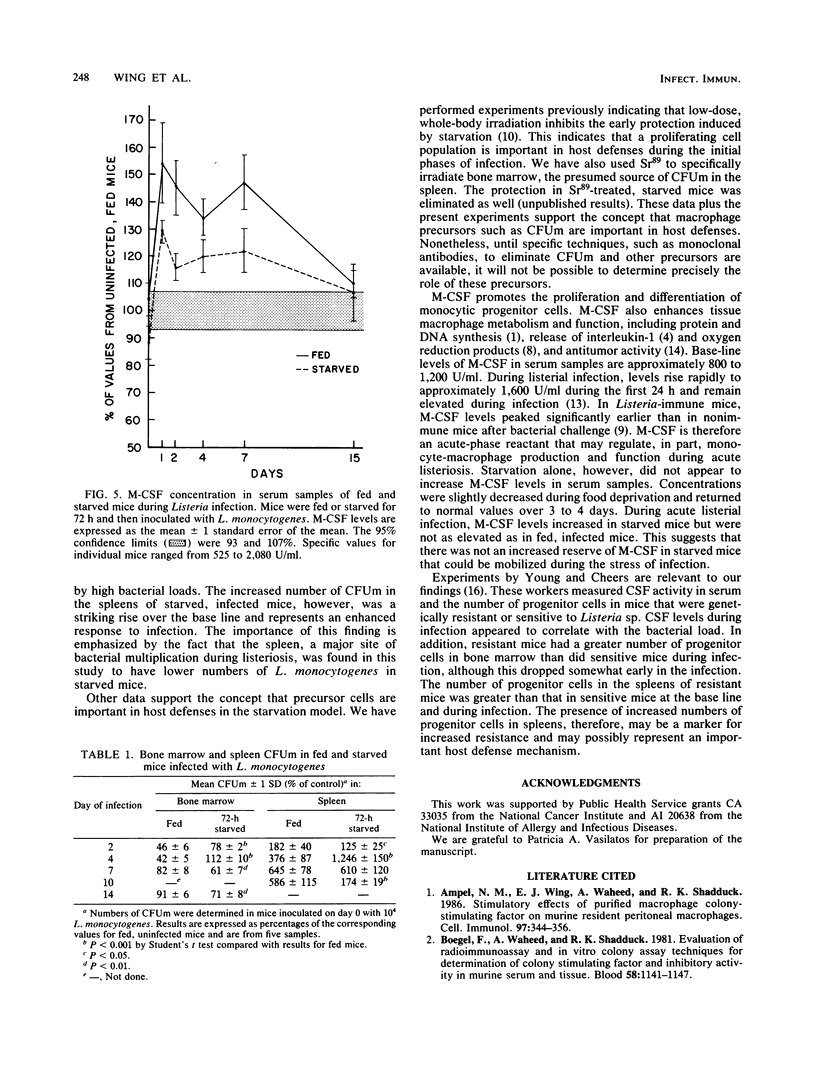
The effect of short-term nutritional deprivation on host defenses and on the parameters of macrophage production was determined in outbred mice. Confirming previous data from this laboratory, initial experiments demonstrated that starved mice were relatively resistant to infection by Listeria monocytogenes as determined by spleen and liver bacterial counts. The number of macrophage progenitor cells in bone marrow rose slightly during a 72-h starvation period and returned to normal during refeeding. By contrast, the number of progenitor cells in spleens fell to 12% of the base line during starvation. The concentration of the macrophage colony-stimulating factor in serum decreased during starvation and returned to normal during refeeding. Additional experiments were performed to determine whether starved mice had increased parameters of macrophage production during listerial infection. The number of progenitor cells in the bone marrow and spleens of starved mice had increased compared with that of fed mice early in infection. Macrophage colony-stimulating factor levels in starved mice rose early and remained elevated during infection but were not as high as in fed mice. These data document the changes in the parameters of monocyte production during starvation and suggest that the number of macrophage progenitor cells may be related to increased resistance to L. monocytogenes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampel N. M., Wing E. J., Waheed A., Shadduck R. K. Stimulatory effects of purified macrophage colony-stimulating factor on murine resident peritoneal macrophages. Cell Immunol. 1986 Feb;97(2):344–356. doi: 10.1016/0008-8749(86)90405-3. [DOI] [PubMed] [Google Scholar]
- Boegel F., Waheed A., Shadduck R. K. Evaluation of radioimmunoassay and in vitro colony assay techniques for determination of colony-stimulating factor and inhibitory activity in murine serum and tissue. Blood. 1981 Dec;58(6):1141–1147. [PubMed] [Google Scholar]
- Moore R. N., Oppenheim J. J., Farrar J. J., Carter C. S., Jr, Waheed A., Shadduck R. K. Production of lymphocyte-activating factor (Interleukin 1) by macrophages activated with colony-stimulating factors. J Immunol. 1980 Sep;125(3):1302–1305. [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadduck R. K., Nagabhushanam N. G. Granulocyte colony stimulating factor. I. Response to acute granulocytopenia. Blood. 1971 Nov;38(5):559–568. [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Pigoli G., Boegel F., Higgins L. Fractionation of antibodies to L-cell colony-stimulating factor by affinity chromatography. Blood. 1979 Jun;53(6):1182–1190. [PubMed] [Google Scholar]
- Wing E. J., Ampel N. M., Waheed A., Shadduck R. K. Macrophage colony-stimulating factor (M-CSF) enhances the capacity of murine macrophages to secrete oxygen reduction products. J Immunol. 1985 Sep;135(3):2052–2056. [PubMed] [Google Scholar]
- Wing E. J., Barczynski L. C., Waheed A., Shadduck R. K. Effect of Listeria monocytogenes infection on serum levels of colony-stimulating factor and number of progenitor cells in immune and nonimmune mice. Infect Immun. 1985 Aug;49(2):325–328. doi: 10.1128/iai.49.2.325-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Barczynski L. K., Boehmer S. M. Effect of acute nutritional deprivation on immune function in mice. I. Macrophages. Immunology. 1983 Mar;48(3):543–550. [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Barczynski L. K. Effect of acute nutritional deprivation on immune function in mice. II. Response to sublethal radiation. Clin Immunol Immunopathol. 1984 Mar;30(3):479–487. doi: 10.1016/0090-1229(84)90033-3. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Kresefsky-Friedman D. Y. Decreased resistance to Listeria monocytogenes in mice injected with killed corynebacterium parvum: association with suppression of cell-mediated immunity. J Infect Dis. 1980 Feb;141(2):203–211. doi: 10.1093/infdis/141.2.203. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K. Changes in serum colony-stimulating factor and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect Immun. 1984 Jul;45(1):180–184. doi: 10.1128/iai.45.1.180-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K., Nagle L. S., Stephenson K. Effect of colony stimulating factor on murine macrophages. Induction of antitumor activity. J Clin Invest. 1982 Feb;69(2):270–276. doi: 10.1172/JCI110449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Young J. B. Acute starvation protects mice against Listeria monocytogenes. Infect Immun. 1980 Jun;28(3):771–776. doi: 10.1128/iai.28.3.771-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. M., Cheers C. Colony-forming cells and colony-stimulating activity during listeriosis in genetically resistant or susceptible mice. Cell Immunol. 1986 Feb;97(2):227–237. doi: 10.1016/0008-8749(86)90393-x. [DOI] [PubMed] [Google Scholar]


