SUMMARY
Whereas advances in the molecular biology of GABAA receptor complex using knock-out and knock-in mice have been valuable in unveiling the structure, composition, receptor assembly, and several functions of different GABAA receptor subtypes, the mechanism(s) underlying benzodiazepine (BZ) tolerance and withdrawal remain poorly understood. Studies using specific GABAA receptor subunit knock-in mice suggest that tolerance to sedative action of diazepam requires long-term activation of α1 and α5 GABAA receptor subunits. We investigated the role of long-term activation of these GABAA receptor subunits during anticonvulsant tolerance using high affinity and high intrinsic efficacy ligands for GABAA receptors expressing the α5 subunit (imidazenil) or α1 subunit (Zolpidem), and a non-selective BZ recognition site ligand (diazepam). We report here that long-term activation of GABAA receptors by zolpidem and diazepam but not by imidazenil elicits anticonvulsant tolerance. Although anticonvulsant cross-tolerance occurs between diazepam and zolpidem, there is no cross-tolerance between imidazenil and diazepam or zolpidem. Furthermore, diazepam or zolpidem long-term treatment decreased the expression of mRNA encoding the α1 GABAA receptor subunit in prefrontal cortex by 43% and 20% respectively. In addition, diazepam but not zolpidem long-term treatment produced a 30% increase in the expression of the α5 GABAA receptor subunit mRNA in prefrontal cortex. In contrast, imidazenil which is devoid of anticonvulsant tolerance does not elicit significant changes in the expression of α1 or α5 GABAA receptor subunit. These findings suggest that long-term activation of GABAA receptors containing the α1 or other subunits but not the α5 receptor subunit is essential for the induction of anticonvulsant tolerance.
Keywords: Anticonvulsant tolerance, GABAA receptors, diazepam, imidazenil, zolpidem, rats
INTRODUCTION
The emergence of tolerance to the various pharmacological actions of BZs and other positive allosteric modulators of GABA action at GABAA receptors occurs after a defined period of protracted administration. The onset of liability to tolerance varies according to the type of benzodiazepine-recognition site ligand (BZ-RS) and the disease being treated. For example, during protracted administration of diazepam, alprazolam, triazolam, lorazepam or flunitrazepam, the classical full positive allosteric modulators of GABA action that have high intrinsic efficacy at α1-, α2-, α3-, and α5-containing GABAA receptor subtypes, sedation is the first pharmacological action to show tolerance, followed by tolerance to the anticonvulsant action, and ultimately the anxiolytic action (Woods et al., 1992; Nutt, 1986; Costa et al., 2001). In contrast, imidazenil, a positive allosteric modulator of GABA action with low intrinsic efficacy at α1 containing GABAA receptors but with a full intrinsic action at α5-containing GABAA receptors and does not elicit sedation or amnesia (Guidotti et al, 2005; Costa and Guidotti, 1996). In addition, imidazenil, perhaps acting at α2 or α3-containing GABAA receptors elicits potent anticonvulsant and anxiolytic actions and fail to show tolerance to anticonvulsant and anxiolytic actions after protracted treatment with large doses in rodents and non-human primates (Auta et al., 1994; 2000).
Several published work support the hypothesis that selective changes in the expression of different α subunits is associated with BZ tolerance (Ali and Olsen, 2001; Bateson, 2002, Costa et al., 2002; Wafford, 2005); however the role of specific subunits in the mechanism of BZ tolerance development is still not clear. Using knock-in mice in which the α1-, α2-, α3- or α5-GABAA receptor subunits were rendered insensitive to diazepam by histidine-arginine point mutation, van Rijnsoever et al (2004) demonstrated that simultaneous and long-term activation of both α1 and α5 GABAA receptor subunit by diazepam is necessary for the development of tolerance to its sedative action. This conclusion was based on the observation that diazepam is effective at reducing motor activity in α5 knock-in mice without showing tolerance to this effect.
The present studies were designed to investigate the role of the α1 and α5 GABAA receptor subunits in the development of anticonvulsant tolerance following protracted administration of selective positive allosteric modulators of GABAA receptors. Therefore we used: a) zolpidem as the selective ligand for α1 subunit-containing GABAA receptors because of its high affinity and intrinsic efficacy at α1-containing GABAA receptors but a 20-fold lower affinity for α2- and α3- and lack of affinity for α5- (Crestani et al., 2000), α4- (Benke et al., 1997), and α6- (Tang et al., 1995) containing GABAA receptors; b) imidazenil as the high affinity and high intrinsic efficacy positive allosteric modulator of GABA action at α5-containing GABAA receptors. However, based on its anticonvulsant and anxiolytic actions it may presumably be active at α2- and α3-containing GABAA receptors but is inactive at GABAA receptors-containing the α1(Guidotti et al., 2005; Costa and Guidotti 1996; Costa et al., 2002), α4 and the α6 subunits (Knoflach et al., 1996); c) diazepam as the non-selective ligand with high intrinsic efficacy at α1, α2-, α3- and α5-containing GABAA receptors ( Mohler et al, 2001; Lagrange et al., 2007; Guidotti et al., 2005; Costa et al., 2002) but inactive at α4- and α6-containing GABAA receptors (Knoflach et al., 1996; Turner et al, 1991) We compared the respective anti-bicuculline tolerance liability of these BZ recognition site ligands after a 14 day of repeated administration to rats. In addition, we correlated the anti-bicuculline tolerance action of these drugs with changes in the expression mRNA encoding for α1 and α5 GABAA receptor subunit since these subunits including the γ2 subunit have consistently been altered during protracted diazepam treatment (Heninger et al., 1990; Primus and Gallager, 1994; Impagnatiello et al., 1996; Longone et al., 1996; Costa et al., 2002). Furthermore, we examined the changes in the expression of these subunits only in the cerebral cortex since the α1 subunit the target for the sedative action of BZs is highly expressed in this brain area (Rudolph et al., 1999).
MATERIALS AND METHODS
Animals, Drugs, and Reagents
Male Fisher 344 rats (Harlan, Indianapolis) weighing 250–300g were housed three per cage and maintained in a 12-hr light/dark cycle with free access to food and water. All experiments were carried out in accordance to the National Institute of Health, Guide for the Care and Use of Laboratory Animals as approved by the Animal Welfare Committee at the University of Illinois at Chicago.
Diazepam and imidazenil were obtained from Hoffman-La Roche (Nutley, NJ); zolpidem was obtained from Synthelabo Recherche (Bagneux, France); Bicuculline from Sigma-Aldrich Co. (Saint Louis, MO); L-655,708 (11,12,13,13a-Tetrahydro-7-methoxy-9-oxo-9H-imidazzo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylic acid, ethyl ester) from TOCRIS Bioscience (Ellisville, MO); Hot Tub DNA polymerase, Human placenta Ribonuclease inhibitor, BglII and [32]dCTP (3000 Ci/mmol; 1 Ci = 37 GBq) were obtained from Amersham Pharmacia. Moloney murine leukemia virus (MMLV) reverse transcriptase, CsCl, guanidine isothiocyanate, and agarose were obtained from invitrogen life technologies (Carlsbad, CA).
Schedule for Long-Term Treatment
Equipotent anti-bicuculline doses of diazepam, imidazenil or zolpidem were suspended in water containing 0.05% Tween-20 and administered in 1 ml volumes by oral gavage three times daily (at approximately 9:00 a.m, 2:00 p.m. and 7:00 p.m.) for 14 days at increasing doses (diazepam: days 1–3, 17.6 µmol/kg; days 4–6, 35.2 µmol/kg; days 7–10 52.8 µmol/kg; and days 11–14, 70.4 µmol/kg; imidazenil: days 1–3, 2.5 µmol/kg, days 4–6, 5.0 µmol/kg, days 7–10, 7.5 µmol/kg, days 11–14, 10 µmol/kg; zolpidem: days 1–3, 48.8 µmol/kg; days 4–6, 97.6 µmol/kg; days 7–10, 146.4 µmol/kg; days 11–14, 195.2 µmol/kg). Control rats received vehicle treatment.
Bicuculline Seizure test
A stock of bicuculline HCl [1 mg/ml (2.7 µmol/ml)] was prepared by dissolving (+)-bicuculline in 0.1 N HCl and then diluted with normal saline (0.9% NaCl) solution to a final concentration of 0.27 µmol/ml (0.1 mg/ml). The convulsive threshold dose of bicuculline was determined by infusing 0.27 µmol/ml of bicuculline into the tail vein of unrestrained rats (250–300 g ) at a constant rate of 0.46 ml/min using a Kd Scientific infusion pump (Model 200, New Hope, PA). The infusion was stopped with the appearance of the first visual sign of tonic-clonic seizures and the time to elicit this event noted. Using the time to elicit tonic-clonic seizures, the infusion rate, and the concentration of bicuculline (0.27 µmol/ml), and the respective weight of each rat, the convulsive threshold dose of bicuculline was determined and expressed as micromoles per kilogram (µmol/kg). The mean (± SEM) threshold dose of bicuculline needed to elicit tonic-clonic seizures was calculated for each group of rats.
Anticonvulsant Tolerance Test
Rats receiving long-term diazepam, imidazenil or zolpidem treatment were left drug-free for at least 18 hr before receiving single oral dose challenge with the respective benzodiazepine receptor ligands or vehicle, followed 30 min later by bicuculline i.v. infusion (seizure test).
RNA Isolation and Quantitative RT-PCR Analysis
The prefrontal cortices of diazepam, imidazenil, zolpidem or vehicle treated rats were removed and frozen on dry ice. Total RNA was isolated as previously described by Impagnatiello et al. 1996. Quantitative RT-PCR measurements were performed using mutated internal standards as previously described by Grayson et al., 1998. Briefly, known and increasing amounts of cRNA derived from the respective GABAA receptor subunit internal standard template (prepared as described by Grayson et al., 1993), were added to a constant amount (1 µg) of total RNA isolated from rat prefrontal cortices. The cRNA/RNA mixtures were denatured for 5 min at 80° and then reverse-transcribed with 200 units of MMLV reverse transcriptase in the presence of dNTPs and 2.5 mM random hexamers in a final volume of 20 µl. The reversed transcribed products were then amplified with Hot Tub DNA polymerase in a Thermal Cycler. Trace amounts of [32]dCTP were added to the reaction mixtures for subsequent quantification. The specific primers for α1 GABAA receptor subunit were: forward, 5’-AGCTATACCCCTAACTTAGCCAGG-3’; reverse, 5’-AGAAAGCGATTCTCAGTGCAGAGG; for the a5 subunit the specific primers were: forward, 5’-CAAGAAGGCCTTGGAAGCAGCTAA-3’; reverse, 5’-GGTTTCCTGTCTTACTTTGGAGAG-3’. The amplification product from both mRNA and cRNA templates, in a post-amplification step, were digested with BglII (cRNA mutation site), and the products were separated by agarose gel electrophoresis. The radioactive counts incorporated into the reversed transcribed and amplified standard cRNA divided by the counts incorporated into the corresponding subunit mRNA amplification product were plotted as a function of the known amount of internal standard cRNA added to the respective test samples. Absolute amounts were determined from the point of equivalence as previously described by Grayson et al. (1993) and Impagnatiello et al. (1996).
RESULTS
Determination of equipotent anti-bicuculline doses of zolpidem, diazepam and imidazenil
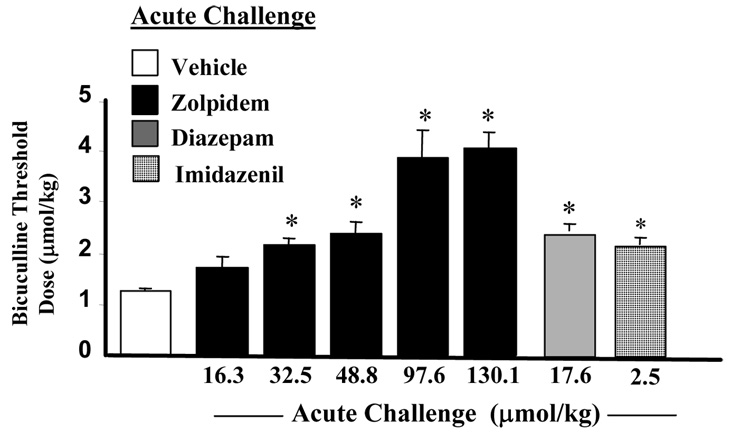
The long-term dosing regimen for diazepam or imidazenil to study anticonvulsant tolerance liability was previously established in our laboratory. In these studies we demonstrated that the threshold dose for bicuculline-induced tonic-clonic convulsions in naïve rats receiving a single oral dose of diazepam (17.6 µmol/kg) or imidazenil (2.5 µmol/kg) 30 min before the start of bicuculline was 2-fold higher than that for the vehicle-treated group (Miyata et al., 1987; Giusti et al., 1993; Auta et al., 1994). We therefore determined the dose of zolpidem that is equipotent to diazepam (17.6µmol/kg) or imidazenil (2.5 µmol/kg) at protecting naïve rats from bicuculline-induce tonic-clonic seizures. The results of these experiments shown in Fig. 1 demonstrate that zolpidem produced a dose-dependent increase in the convulsive threshold dose of bicuculline in naïve rats. Compared to diazepam or imidazenil, larger equimolar doses of zolpidem are required to elicit comparable anti-bicuculline efficacy. Moreover, 48.8 µmol/kg of zolpidem is equipotent to 17.6 µmol/kg of diazepam or 2.5 µmol/kg of imidazenil at increasing the convulsive threshold dose of bicuculline.
Fig. 1.
Acute oral treatment with zolpidem, diazepam or imidazenil increased bicuculline threshold dose for tonic-clonic seizures. Animals received oral administration of zolpidem (16.3 to 130.1µmol/kg), diazepam (17.6µmol/kg), imidazenil (2.5µmol/kg) or vehicle 30 min prior to bicuculline (0.27µmol/ml) infusion. Each bar is the mean ± SEM of five animals. Data were subjected to ANOVA followed by Dunnet’s multiple range test.*p<0.05 compared with vehicle treatment.
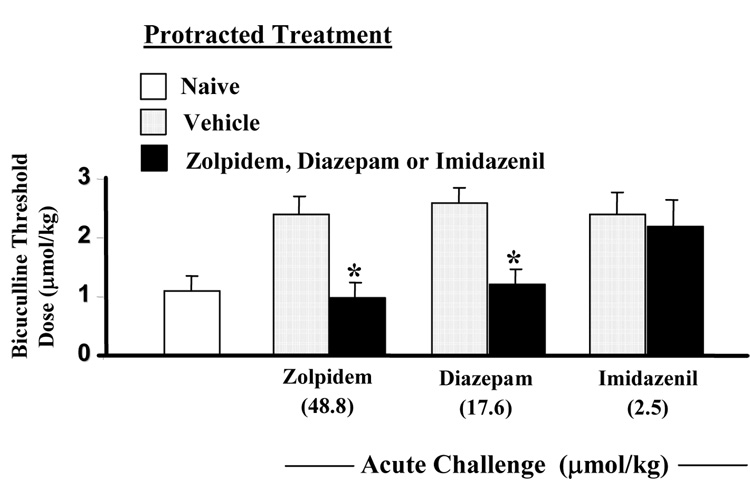
Tolerance to the anti-bicuculline action of zolpidem
To investigate the role of α1 GABAA receptor subunit in anticonvulsant tolerance, we determined the anti-bicuculline action of zolpidem after protracted administration. After 14 days of repeated administration of zolpidem (48.8 to 195.2µmol/kg), the ability of an acute challenging oral dose of zolpidem (48.8 µmol/kg) to increase the convulsive threshold dose of bicuculline was virtually abolished (Fig.2.), suggesting the development of tolerance to the anticonvulsant action of zolpidem. Fig. 2 also shows that 14 days treatment with equipotent dosing regimens of diazepam or imidazenil induces tolerance to the anti-bicuculline action of diazepam but not that of imidazenil. We and others have reported similar results with diazepam and imidazenil in rats and mice (Tietz et al., 1986; Marley and Gallager, 1989, Auta et al., 1994, Ghiani et al., 1994; Impagnatiello et al., 1996; Zanotti et al., 1996; Pesold et al., 1997).
Fig. 2.
Long-term (14 days) treatment with zolpidem or diazepam but not imidazenil results in anticonvulsant tolerance action. Rats treated for 14 days with vehicle or with increasing doses of zolpidem, diazepam, or imidazenil (see method for dosing schedule) were left drug free for 18 h and then received acute oral pretreatment with zolpidem (48.8 µmol/kg), diazepam(17.6µmol/kg) or imidazenil(2.5µmol/kg) 30 min before prior to receiving bicuculline infusion. Each bar is the mean ± SEM for 6 rats. Data were subjected to ANOVA followed by Dunnet’s multiple range test.*p<0.05 versus rats that received 14 days of vehicle treatment.
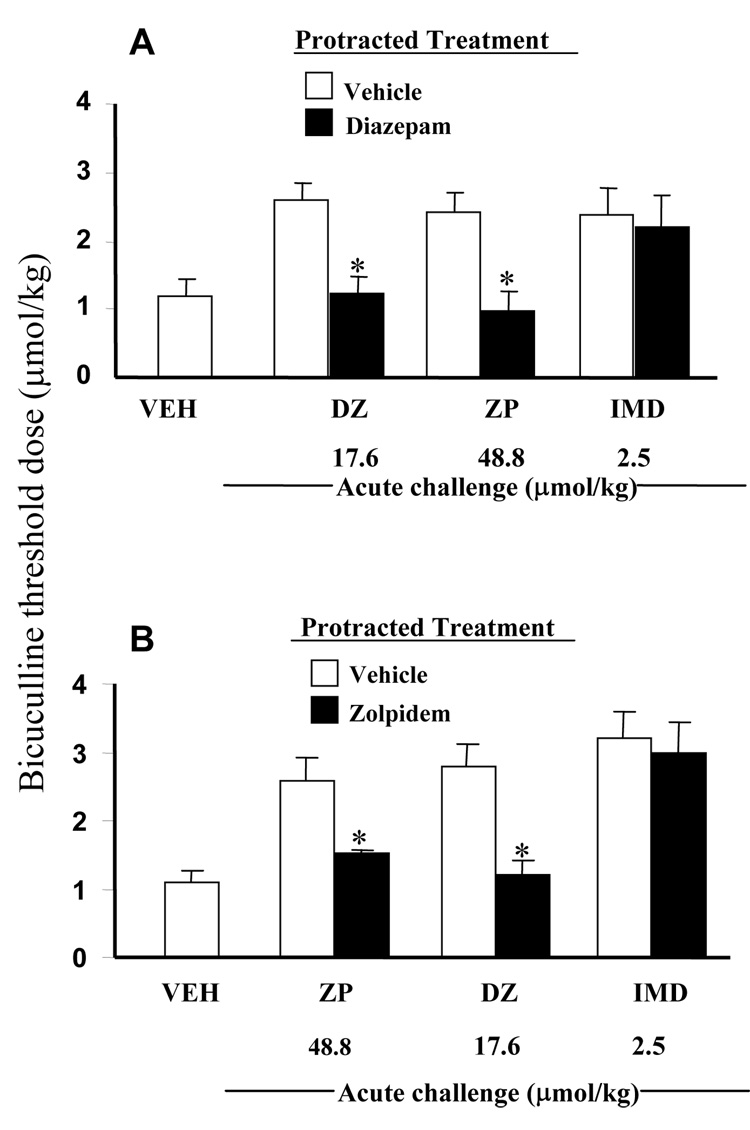
Cross-tolerance between diazepam and zolpidem but not with imidazenil
Rats receiving 14 days of protracted vehicle or diazepam treatment were acutely challenged with vehicle, diazepam, zolpidem, or imidazenil and the threshold dose of bicuculline to elicit tonic-clonic convulsions determined. The result of these experiments shown in Fig. 3A demonstrate that 14 days protracted diazepam treated rats develop tolerance to the anticonvulsant action of single oral doses of diazepam or zolpidem administered 30 min before beginning bicuculline convulsion test. In contrast, 14 days protracted diazepam treated rats did not show tolerance to the anticonvulsant action of a single oral dose of imidazenil. It is noteworthy that the extent of the decrease in the anti-bicuculline action of zolpidem following long-term treatment is comparable to that seen with diazepam long-term treatment. Interestingly, when rats receiving 14 days of protracted vehicle or zolpidem treatment were acutely challenged with zolpidem, diazepam or imidazenil, the anti-bicuculline actions of zolpidem and diazepam but not imidazenil were significantly lower that their respective vehicle groups (Fig. 3B). These results show the presence of cross-tolerance between diazepam and zolpidem and lack of cross-tolerance between diazepam or zolpidem and imidazenil because imidazenil is still effective in rats tolerant to the anticonvulsant action of diazepam or zolpidem.
Fig. 3.
Long-term (14 days) treatment with diazepam (A) or zolpidem (B) resulted to anti-bicuculline cross tolerance between diazepam and zolpidem but not between imidazenil and zolpidem or diazepam. Rats treated for 14 days with diazepam (A, closed bars), zolpidem (B, closed bars) or vehicle (opened bars) were left drug free for 18 h then received acute oral challenge with vehicle, diazepam(17.6µmol/kg), zolpidem(48.8 µmol/kg) or imidazenil(2.5µmol/kg) 30 min prior to receiving bicuculline infusion. Each bar is the mean ± SEM for 6 rats. Data were subjected to ANOVA followed by Dunnet’s multiple range test.*p<0.05 versus respective controls.
Changes in GABAA Receptor Subunit Expression in Prefrontal Cortex of Long-term Diazepam, Zolpidem, and Imidazenil Treated Rats
To investigate the role of GABAA receptor subunits in the development of tolerance to the anticonvulsant action of positive allosteric modulators of GABA action at GABAA receptors, we studied the expression of the α1 and α5 subunits in prefrontal cortex of rat brains that received long-term treatment of vehicle, diazepam, zolpidem and imidazenil. We focused on these two GABAA receptor subunits for two reasons. First, previous studies in our laboratory have consistently shown that 14-day treatment with diazepam down-regulate the expression of the α1 subunit and up-regulate the expression of the α5 subunit in rat prefrontal cortex (Impagnatiello et al., 1996; Pesold et al., 1997; Costa et al., 2003). Second, the finding that simultaneous and chronic activation of the α1 and α5-GABAA receptor subunits is crucial for the development of tolerance to the motor-depressant action of diazepam (van Rijnsoever et al., 2004). Table 1 shows that in rat prefrontal cortex the expression of mRNA encoding for the α1 GABAA receptor subunit is significantly decreased by about 43% and 20% following long-term treatment with diazepam and zolpidem respectively; whereas no significant changes in the expression of either subunit was observed following protracted imidazenil treatment. In contrast, while long-term treatment with diazepam produced a significant increase (about 30%) in the expression of mRNA encoding for the α5-GABAA receptor subunit, long-term treatment with zolpidem or imidazenil did not significantly alter the expression of this subunit in prefrontal cortex. In previous studies (Impagnatiello et al., 1996; Longone et al., 1996; Pesold et al., 1997; Costa et al., 2002) we have shown that protracted administration of imidazenil neither induce tolerance to its anticonvulsant action nor produced significant changes in the expression of GABAA receptor subunit.
TABLE 1.
Quantitative competitive RT-PCR analysis of α1 and α5 GABAA receptor subunit mRNA in prefrontal cortex of rats receiving vehicle, diazepam, zolpidem, or imidazenil long-term treatment
| mRNA Content (attomol/µg of total RNA) | ||||
|---|---|---|---|---|
| GABAA Receptor Subunit | Vehicle | Diazepam | Zolpidem | Imidazenil |
| α1 | 529 ± 53 | 300 ± 18* | 421 ± 22* | 440 ± 61 |
| α5 | 80 ± 9 | 104 ± 6* | 86 ± 7 | 95 ± 8 |
Rats treated for 14 days with vehicle or increasing doses of diazepam, zolpidem, or imidazenil (see methods for dosing schedule) were sacrificed 18 h after administration of the last dose. Prefrontal cortices were dissected out as previously described (Impagnatiello et al., 1997). Each value is the mean ± SEM for six sets of competitive RT-PCR experiments (ANOVA followed by Duncan’s multiple range test).
p<0.05 compared with respective vehicle-treated groups.
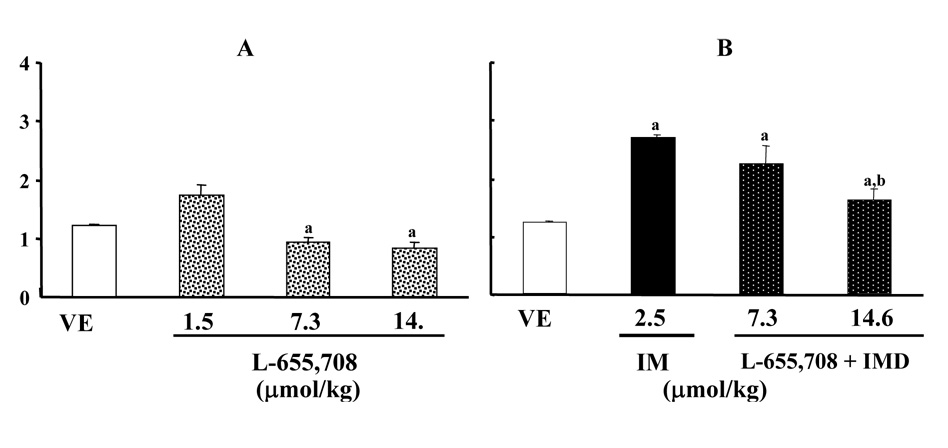
L-655,708 attenuates the anti-bicuculline action of imidazenil
To investigate whether α5-containing GABAA receptors are involved in the anti-bicuculline action of imidazenil, we studied the effect of L-655,708 (a potent and selective inverse agonist that exhibits a 100-fold affinity for α5-containing GABAA receptors when compared to α1 containing receptors; Quirk et al., 1996; Atack et al., 2006) alone and in combination with imidazenil. Fig. 4A shows that a 30 min pretreatment with L-655,708 produced a dose-independent decrease of the bicuculline threshold dose to elicit tonic-clonic seizures. The lowest dose (0.5 mg/kg) tested produced a modest increased whereas the highest doses (2.5 and 5 mg/kg) significantly decreased the bicuculline threshold dose to elicit tonic-clonic seizures. Fig. 4B shows that L-655,708 administered in combination with imidazenil, attenuated the anti-bicuculline action of imidazenil in a dose dependent manner. This data confirmed that the ant-ibicuculline action of imidazenil is in part mediated by its positive modulatory action on α5-containing GABAA receptors.
Fig. 4.
L-655,708 decreased bicuculline threshold dose and antagonize the anti-bicuculline action of imidazenil (IMD). (A) Naïve rats received intraperitoneally (i.p.) injection of vehicle (VEH) or increasing doses of L-655,708 30 min prior to bicuculline (0.27µmol/ml) infusion. (B) Naïve rats received i.p. injection of L-655,708 (7.3 or 14.6 µm/kg) in combination with oral IMD (2.5µmol/kg) 30 min prior to bicuculline (0.27µmol/ml) infusion. Each bar is the mean ± SEM of five animals. Data were subjected to ANOVA followed by Bonferroni t-test: ap<0.001 VEH versus L-655,708, IMD; bp<0.001 IMD versus L-655,708 + IMD.
Although L-655,708 lowered bicuculline threshold dose for tonic-clonic seizures in a dose-independent manner, it attenuated imidazenil anti-bicuculline action in a dose-dependent manner. This finding suggests that the interaction between imidazenil and L-655,708 may be due to a pharmacological antagonism and/or perhaps due to its anxiogenic-like effects (Navarro et al., 2002, 2004) which is probably mediated via its inverse agonist efficacy at α2-, α3-as well as on α5-containing GABAA receptors (Atack et al., 2006).
DISCUSSION
Investigations into the underlying mechanisms for tolerance and/or dependence to the actions of BZ full positive allosteric modulators of GABA action at different GABAA receptor subtypes indicate that these behavioral alterations are related to the pharmacodynamic rather than to the pharmacokinetic characteristics of these drugs (Woods et al., 1992; Auta et al., 1994). Early investigations on the mechanism of BZ tolerance in which electrophysiological recordings were used indicated that long-term treatment with BZ acting as full positive allosteric modulators of GABA action at the majority of GABAA receptor subtypes reduced postsynaptic sensitivity to GABA (Gallager et al., 1984). We have previously shown that the differences in anticonvulsant tolerance liability between diazepam and imidazenil or bretazenil cannot be attributed to differences in drug pharmacokinetics, because brain concentrations of these drugs and their metabolites are similar in both naïve and long-term treated animals (Auta et al., 1994). Thus one can exclude a role for pharmacokinetic variability in the mechanism for tolerance to the anti-bicuculline action of diazepam or zolpidem observed in the present study.
Mice with GABAA receptor subunits point mutations or knock-out have been valuable in studying the importance of GABAA receptor subtypes in the mechanisms underlying tolerance and dependence to the action of different BZs. Using point mutation knock-in mice, it has been reported that GABAA receptors including the α1 subunit mediate the acute sedative (Rudolph et al., 1999; Mckernan et al., 2000) and in part the anticonvulsant (Rudolph et al., 1999) action of diazepam, while the anxiolytic effect is mediated by α2-containing GABAA receptors (Low et al., 2000). The α5 GABAA receptor subunit which is highly expressed in hippocampus, including the CA1 and CA3 regions, plays a significant role in spatial memory performance (Chambers et al., 2003; Rudolph and Mohler, 2004), freezing response in trace but not delay fear conditioning (Crestani et al., 2002b), and in fear conditioning extinction (Yee et al., 2004; Crestani et al., 2002b). In contrast, GABAA receptors including the α2, α3 or α5 subunits that are located on motor neurons and in the dorsal horn of the spinal cord are believed to mediate the muscle relaxant and anti-hyperalgesic actions of diazepam (Bohlhalter et al., 1996; Crestani et al., 2001; Knabl et al., 2008).
The protective action of diazepam in pentylenetetrazole induced tonic-clonic convulsion is significantly reduced in α1 mutated mice when compared to their wild-type counterparts (Rudolph et al., 1999). Hence long-term activation of α1-containing GABAA receptors by zolpidem (a full α1 subunit selective positive allosteric modulator) or diazepam (a full and non-selective positive allosteric modulator) but not the activation of α5-, and presumably α2- or α3-containing GABAA receptors by imidazenil (which is inactive at α1-containing GABAA receptors) might underlie the mechanism for anticonvulsant tolerance. In fact, our results suggest that the down-regulation of the α1 receptor subunit that ensues following long-term diazepam or zolpidem treatment might partly be responsible for the anticonvulsant tolerance and/or cross tolerance to acute challenge with diazepam or zolpidem. Imidazenil neither induces tolerance to its anticonvulsant action nor changes the expression of α1 GABAA receptor subunit after long-term treatment. In addition, imidazenil does not show cross tolerance to diazepam or zolpidem; therefore it is likely that the anticonvulsant action of imidazenil is mediated by α-containing GABAA receptors that do not include the α1 subunit and also independent from the decreased expression of α1 or other GABAA receptor subunits that are caused by diazepam or zolpidem. The antagonism of imidazenil anti-bicuculline action by L-655,708, the proposed selective inverse agonist for α5-containing GABAA receptors, further suggests that the anticonvulsant action of imidazenil is in part mediated by an action on α5- but we cannot exclude an action on α2- or α3-containing GABAA receptors.
It has also been reported that tolerance to the motor-depressant action of diazepam requires the simultaneous activation of α1 and α5 GABAA receptor subunits (van Rijnsoever et al., 2004). Our study with imidazenil (full agonist at α5 but inactive at α1), zolpidem (full agonist at α1 but inactive at α5), and diazepam (full agonist at α1 and α5-containing GABAA receptors) suggest that tolerance to the anticonvulsant action of diazepam and zolpidem might not require or depend on the long-term and simultaneous activation of GABAA receptors containing the α1 and α5 subunits. It is important to note that while the present studies relied on the selectivity and differential intrinsic efficacies of three BZ recognition site ligands, the studies by van Rijnsoever et al (2004) was based on the sensitivity of knock-in mice in which the α1-, α2-, α3- or α5 GABAA receptor subunits had been rendered insensitive to diazepam by histidine-arginine single point mutation. It is also interesting to note that differential neuronal adaptive responses in the expression of GABAA receptor subunits have been reported following GABAA receptor subunits knock-in or knock-out (Sur et al., 2001; Kralic et al.; Crestani et al., 2002b).
The results of the present studies strongly suggests that long-term activation of GABAA receptors containing the α1 but not the α5 subunit by BZ positive allosteric modulators endowed with high intrinsic efficacy at these receptor subtypes is essential for the development of anticonvulsant tolerance. This inference is based on the observation that cross-tolerance exist between zolpidem and diazepam, two full positive allosteric modulators that potentiate GABA action with high intrinsic efficacy at α1-containing GABAA receptors. A recent report by Knabl et al (2008) demonstrated that long-term treatment with L-838,417, a non-sedating α1-sparing benzodiazepine recognition site ligand but a partial agonist at α2-, α3- and α5-containing GABAA receptors fail to show tolerance to its analgesic effect. Moreover, in prefrontal cortex, the expression of the α1 subunit significantly decreased after diazepam or zolpidem protracted treatment while the expression of the α5 fail to change after zolpidem or imidazenil protracted treatment but significantly increased after diazepam protracted treatment. This finding further suggests the important role of theα1 subunit and the lack of a significant role for the α5 subunit in the development of tolerance to the anticonvulsant actions of BZ positive allosteric modulators.
In summary, we have shown that in prefrontal cortex, the expression of mRNA encoding for the α1 GABAA receptor subunit decreased by about 43% and 20% in rats treated for 14 days with diazepam and zolpidem respectively. In addition, long-term treatment with diazepam but not zolpidem or imidazenil is associated with a 30 % increase in the expression of the α5 GABAA receptor subunit. These results indicate that long-term activation of α1-containing GABAA receptors by high efficacy positive allosteric modulators of GABA action at these receptor subtypes down-regulate the sensitivity of α1-containing GABAA receptor subunits to GABA which consequently leads to the development of anticonvulsant tolerance. In contrast, imidazenil which is virtually unable to potentiate GABA action at α1- (Costa et al., 2002), α4- and α6-containing (Knoflach et al., 1996) GABAA receptors but elicits long-term activation of α5-containing GABAA receptors Guidotti et al., (2005) is devoid of anticonvulsant tolerance following protracted administration. Furthermore, imidazenil is still effective as an anticonvulsant in animals tolerant to both diazepam and zolpidem, probably because of its high affinity and intrinsic efficacy at α5- and most likely α2 or α3-containing GABAA receptors. Thus, the development of a new generation of BZ recognition site ligands that specifically target selective GABAA receptor subtypes especially in the single point mutation mutant mice will further elucidate the physiology and pharmacology of different GABAA receptor subtypes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ali NJ, Olsen RW. Chronic benzodiazepine treatment of cells expressing recombinant GABAA receptors uncouples allosteric binding: studies on possible mechanisms. Journal of Neurochemistry. 2001;79:1100–1108. doi: 10.1046/j.1471-4159.2001.00664.x. [DOI] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology. 2006;51(6):1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Auta J, Giusti P, Guidotti A, Costa E. Imidazenil, a positive allosteric modulator of GABAA receptors exhibits low tolerance and dependence liabilities. Journal of Pharmacology and Experimental Therapeutics. 1994;270:1262–1269. [PubMed] [Google Scholar]
- Auta J, Guidotti A, Costa E. Imidazenil prevention of alprazolam-induced acquisition deficit in Patas monkeys is devoid of tolerance. Proceeding of the National Academy of Science USA. 2000;97(5):2314–2319. doi: 10.1073/pnas.97.5.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson AN. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Current Pharmaceutical Design. 2002;8:5–12. doi: 10.2174/1381612023396681. [DOI] [PubMed] [Google Scholar]
- Benke D, Michael C, Mohler H. GABAA receptors containing the α4-subunit: prevalence, distribution, pharmacology, and subunit architecture in situ. Journal of Neurochemistry. 1997;69(2):806–814. doi: 10.1046/j.1471-4159.1997.69020806.x. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. Laminar compartmentalization of GABAA receptor subtypes in the spinal cord: An immunohistochemical study. Journal of Neuroscience. 1996;16(1):283–297. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Broughton HB, Collison N, Cook S, Dawson GR. Identification of a novel, selective GABAA alpha5 receptor inverse agonist which enhances cognition. Journal of Neuroscience. 2003;46:2227–2240. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- Costa E, Guidotti A. Benzodiazepines on trial: a research strategy for their rehabilitation. Trends in Pharmacological Sciences. 1996;17:192–200. doi: 10.1016/0165-6147(96)10015-8. [DOI] [PubMed] [Google Scholar]
- Costa E, Auta J, Grayson DR, Matsumoto K, Pappas DG, Zhang X, Guidotti A. GABAA receptors and benzodiazepines: a role for dendritic resident mRNAs. Neuropharmacology. 2002;43:925–937. doi: 10.1016/s0028-3908(02)00199-5. [DOI] [PubMed] [Google Scholar]
- Costa E, Grayson DR, Mitchell CP, Tremolizzo L, Veldic M, Guidotti A. GABAergic cortical neuron chromatin as a putative target to treat schizophrenia vulnerability. Critical Reviews in Neurobiology. 2003;15:121–142. doi: 10.1615/critrevneurobiol.v15.i2.20. [DOI] [PubMed] [Google Scholar]
- Costa E, Auta J, Guidotti A. Tolerance and dependence to ligands of benzodiazepine recognition sites expressed by GABAA receptors. In: Mohler H, editor. Handbook of Experimental Pharmacology. vol. 50. 2001. pp. 227–250. [Google Scholar]
- Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. British Journal of Pharmacology. 2000;131:1251–1254. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Low K, Keist R, Mandelli M, Mohler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Molecular Pharmacology. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- Crestani F, Assandri R, Tauber M, Martin JR, Rudolph U. Contribution of the β1-GABAA receptor subtype to the pharmacological actions of benzodiazepines site inverse agonists. Neuropharmacology. 2002a;43:679–684. doi: 10.1016/s0028-3908(02)00159-4. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal β5 GABAA receptors. Proceeding of the National Academy of Science USA. 2002b;99(13):8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallager DW, Lakowski JM, Gonsalves SF, Rauch SL. Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature. 1984;308:74–77. doi: 10.1038/308074a0. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Serra M, Motzo C, Giusti P, Cuccheddu T, Porceddu ML, Biggio G. Chronic administration of an anticonvulsant dose of imidazenil fails to induce tolerance of GABAA receptor function in mice. European Journal of Pharmacology. 1994;254:299–302. doi: 10.1016/0014-2999(94)90470-7. [DOI] [PubMed] [Google Scholar]
- Giusti P, Ducic I, Puia G, Arban R, Walser A, Guidotti A, Costa E. Imidazenil: A new partial positive allosteric modulator of GABA action at GABAA receptors. Journal of Pharmacology and Experimental Therapeutics. 1993;266:1018–1028. [PubMed] [Google Scholar]
- Grayson DR, Bovolin P, Santi MR. Absolute quantitation of γ-aminobutyric acid a receptor subunit messenger RNAs by competitive polymerase chain reaction. Methods in Neuroscience. 1993;12:191–208. [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: a new treatment target on the horizon. Psychopharmacology(Berlin) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Heninger C, Saito N, Tallman JF, Garrett MP, Vitek KM, Duman RS, Gallager DW. Effects of continuous diazepam administration on GABAA subunit mRNA in rat brain. Journal of Molecular Neuroscience. 1990;2:101–107. doi: 10.1007/BF02876917. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Pesold C, Longone P, Caruncho HJ, Fritschy JM, Costa E, Guidotti A. Modifications of γ-aminobutyric acidA receptor subunit expression in rat neocortex during tolerance to diazepam. Molecular Pharmacology. 1996;49:822–831. [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hosl K, Reinold H, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–335. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Luddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharamacological modulation of diazepam-insensitive recombinant γ-aminobutyric acidA receptors α2β2γ2 and α6β2γ2. Molecular Pharmacology. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Kralic JE, Korpi ER, O’Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABAA receptor α1 subunit knockout mice. Journal of Pharmacology and Experimental Therapeutics. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of α4 subtype-containing GABAA receptors to synaptic and extra synaptic GABA. Journal of Physiology. 2007;578(Pt3):655–676. doi: 10.1113/jphysiol.2006.122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longone P, Impagnatiello F, Guidotti A, Costa E. Reversible modification of GABAA receptor subunit mRNA expression during tolerance to diazepam-induced cognition dysfunction. Neuropharmacology. 1996;35:1465–1473. doi: 10.1016/s0028-3908(96)00071-8. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolp U. Molecular and neural substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Marley RJ, Gallager DW. Chronic diazepam treatment produces regional specific changes in GABA-stimulated chloride influx. European Journal of Pharmacology. 1989;159:217–223. doi: 10.1016/0014-2999(89)90151-9. [DOI] [PubMed] [Google Scholar]
- Mckernan RM, Rosahl TM, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garret L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro Jl, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nature Neuroscience. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Miyata M, Mocchetti I, Ferrarese C, Guidotti A, Costa E. Protracted treatment with diazepam increases the turnover of putative endogenous ligands for the benzodiazepine/β carboline recognition site. Proceeding of the National Academy of Science USA. 1987;84(5):1444–1448. doi: 10.1073/pnas.84.5.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H, Crestani F, Rudolph U. GABAA-receptor subtypes: a new pharmacology. Current Opinion in Pharmacology. 2001;1:22–25. doi: 10.1016/s1471-4892(01)00008-x. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Buron E, Martin-Lopez M. Anxiogenic-like activity of L-655,708, a selective ligand for the benzodiazepine site of GABAA receptors which contain the alpha-5 subunit, in the elevated plus-maze test. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2002;26:1389–1392. doi: 10.1016/s0278-5846(02)00305-6. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Buron E, Martin-Lopez M. Behavioral profile of L-655,708, a selective ligand for the benzodiazepine site of GABAA receptors which contain the alpha-5 subunit, in social encounters between male mice. Aggressive Behavior. 2004;30:319–325. [Google Scholar]
- Nutt DJ. Benzodiazepine dependence in the clinic: A cause for anxiety? Trends in Pharmacological Sciences. 1986;7:457–460. [Google Scholar]
- Pesold C, Caruncho HJ, Impagnatiello F, Berg MJ, Fritschy JM, Guidotti A, Costa E. Tolerance to diazepam and changes in GABAA receptor subunit expression in rat neocortical areas. Neuroscience. 1997;79(2):477–487. doi: 10.1016/s0306-4522(96)00609-4. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Gallager DW. GABA receptor subunit mRNA levels are differentially influenced by chronic FG 7142 and diazepam exposure. European Journal of Pharmacology. 1992;226:21–28. doi: 10.1016/0922-4106(92)90078-a. [DOI] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology. 1996;35(9–10):1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bleuthmann H, Mohler H. Benzodiazepine action mediated by specific gamma-aminobutyric acid (A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepine and general anesthetics through mouse genetics. Annual Review of Pharmacology and Toxicology. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidgex F, Macaulay A, Collinson N, O’Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW. Loss of the major GABAA receptor subtype in the brain is not lethal in mice. Journal of Neuroscience. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, Smith MW, Carter DB, Im WB, Vonvoigtlander PF. U-90042, a sedative/hypnotic compound that interacts differentially with GABAA receptor subtypes. Journal of Pharmacology and Experimental Therapeutics. 1995;275:761–767. [PubMed] [Google Scholar]
- Tietz EI, Rosebenberg HC, Chiu TH. Autoradiographic localization of benzodiazepine receptor downregulation. Journal of Pharmacology and Experimental Therapeutics. 1986;236:284–291. [PubMed] [Google Scholar]
- van Rijinsoever C, Tauber M, Choulli MK, Keist R, Rudolph U, Mohler H, Fristschy JM, Crestani F. Requirement of α5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. Journal of Neuroscience. 2004;24(30):6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA. GABAA receptor subtypes: any clue to benzodiazepine dependence? Current Opinion in Pharmacology. 2005;5:47–52. doi: 10.1016/j.coph.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Woods J, Katz J, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacology Review. 1992;44:151–347. [PubMed] [Google Scholar]
- Yee BK, Hauser J, Doglov VV, Keist R, Mohler H, Rudolph U, Feldon J. GABAA receptors containing the α5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. European Journal of Neuroscience. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- Zanotti A, Mariot R, Contarino A, Lipartiti M, Giusti P. Lack of anticonvulsant tolerance and benzodiazepine receptor down-regulation with imidazenil in rats. British Journal of Pharmacology. 1996;117(4):647–652. doi: 10.1111/j.1476-5381.1996.tb15239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]