Abstract
Evidence from multiple laboratories has suggested the possibility that defective membrane recruitment, triggered by mutations in conserved lipid binding domains, could be a common molecular mechanism underlying carcinogenesis. Now a recent paper by Carpten et al. in Nature has identified and analyzed one such mutation; specifically, E17K in the lipid binding pocket of the Akt plextrin homology (PH domain). This study is a tour de force that (i) pinpoints a mutation widespread in human cancers, (ii) analyzes the effect of this mutation on lipid binding domain structure, (iii) shows that the mutation enhances plasma membrane recruitment, and (iv) demonstrates that such recruitment is linked to Akt pathway superactivation, cellular transformation and tumor formation. Overall, the work provides the most convincing illustration to date that a mutation altering the membrane docking of a lipid binding domain can directly trigger cancer. Furthermore, the findings raise intriguing questions regarding the mechanism by which the highly carcinogenic E17K mutation drives enhanced recruitment of the Akt PH domain to the plasma membrane.
The Carpten et al study (1) was carried out by a team of 24 scientists led by Drs. Kerry L. Blanchard and James E. Thomas at Eli Lilly & Company, and by Dr. John Carpten of Translational Genomics Research Institute. The study focuses on the protein kinase Akt, also known as protein kinase B, which is an important element of a membrane-associated signaling pathway regulated by the signaling lipid phosphatidylinositol-3,4,5-trisphosphate (PIP3) [reviewed in (2–6)]. This lipid is produced in the cytoplasmic leaflet of the plasma membrane by the phosphatidylinositol-3-kinase (PI3K) family of lipid kinases, which phosphorylate the substrate lipid phosphatidylinositol-4,5-bisphosphate to generate PIP3. The steady state level of PIP3 is increased by the oncogene Ras, which binds to PI3K and stimulates its kinase activity. The resulting PIP3 recruits an array of proteins possessing PIP3-specific PH domains, including Akt and phosphoinositide-dependent kinase 1 (PDK1), to the plasma membrane. The simultaneous presence of Akt and PDK1 on the membrane surface increases the rate at which PDK1 phosphorylates Akt at a specific regulatory site essential for phospho-activation. Ultimately, the PIP3 signal is degraded by phosphatases, especially by PTEN which hydrolyzes PIP3 back to phosphatidylinositol-4,5-bisphosphate.
Defects in PIP3 signaling play a central role in many cancers. For example, a large number of carcinogenic mutations have been described in Ras, PI3K, Akt, and PTEN (6–10). Notably, a subset of these cancer-linked mutations are located in conserved PH domains and C2 domains, both of which function as lipid binding domains, (1, 7, 11). Before the Carpten et al. study, however, no such mutation in the Akt PH domain had yet been reported.
The PH domain of Akt is a representative example of the conserved PH motif, both structurally and functionally (12–14). The domain possesses a β-sandwich core in which two β-sheets associate to form a structure resembling a flattened β-barrel. At one edge of the β-sandwich a lone α-helix packs against the inter-strand loops, while at the opposite edge the inter-strand loops form a ligand binding pocket. In the case of Akt PH domain, this binding pocket exhibits high affinities for PIP3 and phosphatidylinositol-3,4-bisphosphate (15). PIP3 is believed to be the more important physiological target due to its greater abundance in plasma membrane. At the same time, the affinity of this PH domain for phosphatidylinositol-4,5-bisphosphate, the most abundant PIP lipid in the plasma membrane, is much lower (15). Such low affinity for this common PIP lipid enables Akt to remain in the cytoplasm until a PIP3 signaling event recruits it to the plasma membrane.
To directly test whether the kinase domain or the PH domain of Akt is most important in carcinogensis, Caprten et al searched genomic DNAs isolated from 162 patients with breast, colon, or ovarian cancers for mutations in the Akt gene (1). No mutations were found in the kinase domain, but the PH domain mutation E17K was observed in 9 of the 162 cases, implying that this PH domain defect is present in a significant percentage of human cancers (breast 8%, colon 6%, and ovarian 2%; (1)).
To determine the effects of E17K on PH domain structure, the authors solved the crystal structures of isolated E17K Akt PH domain in both its apo state and in its PIP3-occupied state, where a soluble headgroup analogue (inositol-1,3,4,5-tetraphosphate, or IP4) substitutes for the full PIP3 molecule (1). In the apo site, the replacement of Glu 17 with Lys disrupts a salt bridge, which could, in principle, energetically destabilize the apo state. In the IP4-occupied site, the mutant Lys side chain forms new hydrogen bonds which may stabilize IP4 in the binding pocket.
Thus, in the simplest hypothesis, the E17K mutation is predicted to increase the equilibrium affinity of Akt PH domain for its target PIP3 lipid. Although no direct affinity measurements were carried out, the E17K mutation did cause both the isolated PH domain and full-length Akt to target more efficiently to plasma membrane in cells than their wild type counterparts, especially in the absence of stimulation where mutant Akt was approx. 4-fold more likely to be membrane associated.
Notably, when Carpten et al isolated full length E17K Akt from cells, it exhibited significantly higher specific kinase activity than wild type Akt (1). This superactivation was correlated with a higher level of phosphorylation at its phospho-activation sites relative to WT, while in vitro phosphorylation of the mutant and WT proteins to the same level yielded indistinguishable substrate affinities and Vmax values. When E17K Akt was introduced to cells, it was found to generate high rates of transformation in tissue culture, and to induce leukemia in mice. Since Akt is phosphorylated primarily when bound to plasma membrane, and the mutant spends more time in the membrane-bound state, the simplest hypothesis that explains all of these observations is that the enhanced membrane recruitment caused by E17K is directly responsible for Akt superactivation, cellular transformation, and carcinogenesis.
One question that remains for future study is the molecular mechanism of enhanced membrane recruitment by the E17K mutation. It is not yet clear whether the enhanced plasma membrane targeting is driven by an increased affinity for PIP3, or by an increased affinity for the more abundant phosphatidylinositol-4,5-bisphosphate, representing a loss of target selectivity. Moreover, it is not known whether the putative enhanced affinity for PIP2 or PIP3 arises from a higher on-rate or lower off-rate, or both. The PIP3 target is a rare component of the plasma membrane, even during a peak signal, and it has recently been shown that a PIP3-specific PH domain similar to Akt PH possesses an electrostatic search mechanism that speeds its association with this rare target on the anionic surface of the plasma membrane (16). Thus, the additional positive charge provided by E17K could increase the PIP lipid on-rate, either by enhancing the efficiency of the electrostatic search mechanism used to find the target head group, or by enhancing the rate of PIP lipid association once the head group is found. Alternatively, the cationic, mutant Lys side chain could decrease the rate of dissociation from bound PIP lipid via a direct contact or through-space electrostatic interaction with the phosphates on the PIP headgroup.
In principle, however, it remains possible that the enhanced E17K Akt membrane recruitment arises from an increase in plasma membrane PIP3 or phosphatidylinositol-3,4-bisphosphate levels, rather than from an increase in PIP lipid affinity. At least in certain cells, PIP3 production is regulated by a positive feedback loop involving Rac, PI3K, actin, and Ca2+ (17). If E17K Akt can stimulate PIP3 or phosphatidylinositol-3,4-bisphosphate production, by upregulating a positive feedback loop or some other mechanism, the additional target lipid would recruit more Akt. The observation of Carpten et al that E17K Akt is efficiently recruited to the plasma membrane of unstimulated cells (1), which normally possess very low levels of its target PIP lipids, lends credibility to the possibilities that E17K Akt either binds the abundant phosphatidylinositol-4,5-bisphosphate more tightly, or that the mutant protein somehow stimulates target PIP lipid production. Further equilibrium and kinetic studies comparing the membrane docking reactions of WT and E17K PH domains are needed to clarify the mechanism of enhanced membrane recruitment.
Finally, on a more general note, mutations which modify the membrane recruitment of lipid binding domains likely play a more widespread role in carcinogenesis than previously realized. In the PIP3 signaling pathway alone, lipid binding domains play an essential role in the membrane recruitment of PI3K (C2 domain), PDK1 (PH domain), and PTEN (C2 domain) as well as Akt (PH domain). Mutations linked to cancer have now been observed in all of these domains except for PDK1 PH domain (1, 7, 11). In proteins that upregulate the PIP3 pathway, like PI3K, PDK1 and Akt, mutations that increase membrane recruitment are predicted to be carcinogenic. In proteins that downregulate the PIP3 pathway, like the lipid phosphatase PTEN (a tumor suppressor), mutations that decrease membrane recruitment are predicted to be carcinogenic. In short, defective membrane recruitment – either excessive recruitment or inhibition of recruitment – is likely to be a common molecular mechanism underlying a wide array of human cancers.
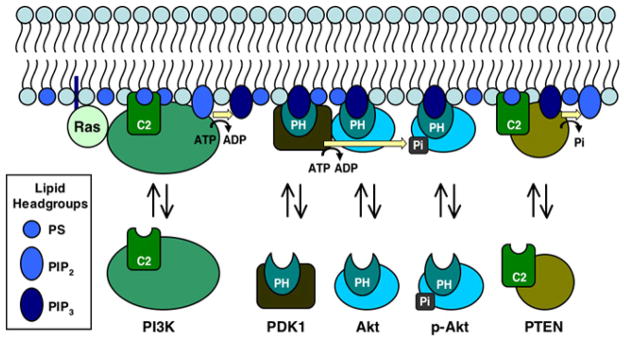
Figure 1. Membrane recruitment by PH and C2 domains in the Ras-PI3K-Akt-PDK1-PTEN signaling pathway.
[Reviewed by (2–6)]. The pathway is triggered by Ras activation of membrane-bound phosphatidylinositol-3-kinase (PI3K) at the surface of the plasma membrane. In turn, PI3K phosphorylates the substrate lipid phosphatidylinositol-4,5-bisphosphate (PIP2), yielding the product signaling lipid phosphatidylinositol-3,4,5-trisphosphate (PIP3). Both Akt/protein kinase B (Akt) and phosphoinositide-dependent kinase 1 (PDK1) possess pleckstrin homology (PH) domains that specifically bind PIP3, thereby recruiting these kinases to the plasma membrane where PDK1 phosphorylates Akt at one or more specific sites required for activation of Akt kinase. Ultimately, the PIP3 signal is degraded by the membrane-bound phosphatase PTEN, yielding PIP2. The E17K mutation in the PIP3 binding pocket of Akt PH domain enhances membrane recruitment (1), and leads to higher than native levels of phospho-activated Akt. Both PI3K and PTEN possess C2 domains believed to be involved in the plasma membrane recruitment of these enzymes (18, 19). Such C2 domains often bind lipids in a Ca2+-dependent or -independent manner and serve as membrane targeting modules (20, 21). Although the PI3K and PTEN C2 domains have both been reported to dock to phospholipid vesicles (18, 22), high affinity target lipids have not yet been identified for these domains.
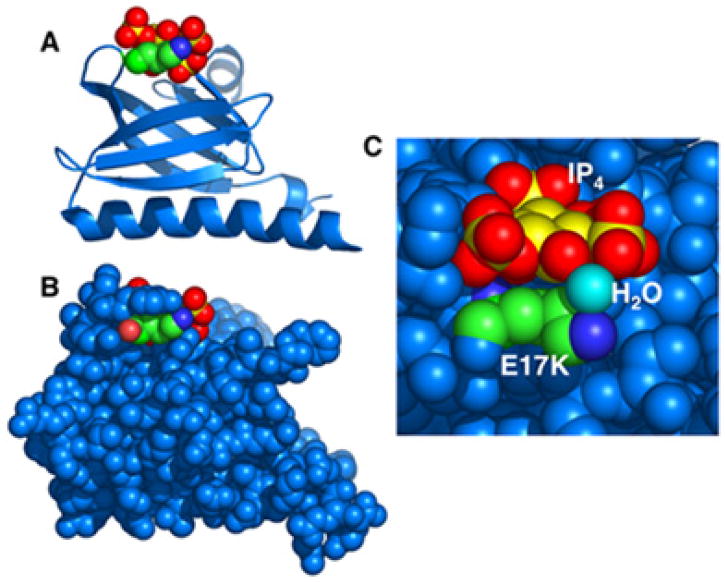
Figure 2. Structural features of the E17K Akt mutant PH domain.
A) and B) Ribbon and CPK renderings, respectively, of the mutant PH domain, illustrating the classic β-sandwich architecture of the domain core, the standard α-helix at one edge of the core, and the PIP-lipid binding loops at the opposite edge (1). Highlighted are the lysine side chain of the E17K mutation (green, with blue nitrogen), as well as a bound IP4 molecule (gold, with red oxygens; a soluble headgroup analogue of PIP3) located in the PIP3 binding pocket. In these two views, the scale and perspective are the same, and the domain is viewed looking sideways into the PIP3 binding pocket. C) Detailed top view looking down into the PIP3 binding pocket, where Tyr 18 has been removed to allow a clear image of both the E17K side chain and a water oxygen (cyan) bridging the mutant lysine amino group (dark blue) to IP4 oxygens (red). Figures generated in Mac PyMol (23).
Acknowledgments
Support provided by NIH grant R01 GM063235 to JJF. The author thanks Drs. James Thomas and Kerry Blanchard for helpful comments on the manuscript.
References
- 1.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, Uhlik M, Lin A, Du J, Qian YW, Zeckner DJ, Tucker-Kellogg G, Touchman J, Patel K, Mousses S, Bittner M, Schevitz R, Lai MH, Blanchard KL, Thomas JE. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 2.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 3.Scheid MP, Woodgett JR. Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett. 2003;546:108–12. doi: 10.1016/s0014-5793(03)00562-3. [DOI] [PubMed] [Google Scholar]
- 4.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–9. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 5.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 6.Der CJ, Van Dyke T. Stopping ras in its tracks. Cell. 2007;129:855–7. doi: 10.1016/j.cell.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Gymnopoulos M, Elsliger MA, Vogt PK. Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci U S A. 2007;104:5569–74. doi: 10.1073/pnas.0701005104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 9.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 10.Steelman LS, Bertrand FE, McCubrey JA. The complexity of PTEN: mutation, marker and potential target for therapeutic intervention. Expert Opin Ther Targets. 2004;8:537–50. doi: 10.1517/14728222.8.6.537. [DOI] [PubMed] [Google Scholar]
- 11.Georgescu MM, Kirsch KH, Kaloudis P, Yang H, Pavletich NP, Hanafusa H. Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res. 2000;60:7033–8. [PubMed] [Google Scholar]
- 12.Thomas CC, Deak M, Alessi DR, van Aalten DM. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr Biol. 2002;12:1256–62. doi: 10.1016/s0960-9822(02)00972-7. [DOI] [PubMed] [Google Scholar]
- 13.DiNitto JP, Cronin TC, Lambright DG. Membrane recognition and targeting by lipid-binding domains. Sci STKE 2003. 2003:re16. doi: 10.1126/stke.2132003re16. [DOI] [PubMed] [Google Scholar]
- 14.Lemmon MA. Pleckstrin homology (PH) domains and phosphoinositides. Biochem Soc Symp. 2007:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frech M, Andjelkovic M, Ingley E, Reddy KK, Falck JR, Hemmings BA. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J Biol Chem. 1997;272:8474–81. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 16.Corbin JA, Dirkx RA, Falke JJ. GRP1 pleckstrin homology domain: activation parameters and novel search mechanism for rare target lipid. Biochemistry. 2004;43:16161–73. doi: 10.1021/bi049017a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans JH, Falke JJ. Pro Nat Acad Sci USA. 2007. Ca2+ influx is an essential component of the positive feedback loop that maintains leading edge structure and activity in macrophages. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker EH, Perisic O, Ried C, Stephens L, Williams RL. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature. 1999;402:313–20. doi: 10.1038/46319. [DOI] [PubMed] [Google Scholar]
- 19.Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–34. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 20.Cho W, Stahelin RV. Membrane binding and subcellular targeting of C2 domains. Biochim Biophys Acta. 2006;1761:838–49. doi: 10.1016/j.bbalip.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das S, Dixon JE, Cho W. Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci U S A. 2003;100:7491–6. doi: 10.1073/pnas.0932835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delano WL. The PyMol molecular graphics system, DeLano Scientific. 2002 World Wide Web http://www.pymol.org.