Abstract
Background
Here we explored the possibility that the opportunistic pathogen, Pseudomonas aeruginosa (P.a) senses low phosphate (Pi) as a signal of host injury and shifts to a lethal phenotype.
Methods
Virulence expression in P. aeruginosa was examined in vitro under low phosphate conditions by assessing expression of the PA-I lectin, a barrier dysregulating protein, pyocyanin and biofilm production, and PstS, a phosphate scavenging protein. Virulence expression in vivo was assessed using surgically injured mice (30% hepatectomy) intestinally inoculated with P. aeruginosa (P.a).
Results
In vitro experiments demonstrated that acute phosphate depletion resulted in a significant (p=0.001) increase in the expression the PA-I lectin, biofilm, pyocyanin, and PstS. Surgical injury caused significant (p=.006) depletion of intestinal phosphate concentration and increased mortality (60%) due to intestinal P.a which was completely prevented with oral phosphate supplementation and restoration of intestinal phosphate- neither of which were observed with systemic (IV) administration. PstS gene expression was 32-fold higher in P.a recovered from the cecum following hepatectomy indicating inadequate intestinal Pi.
Conclusions
Surgical injury- induced intestinal phosphate depletion shifts the phenotype of P. aeruginosa to express enhanced virulence in vitro and lethality in vivo. Intestinal phosphate repletion may be a novel strategy to contain pathogens associated with lethal gut-derived sepsis.
Introduction
Work from our laboratory has established that following surgical injury, host products of physiologic stress are released into the intestinal tract that directly activate the molecular circuitry of colonizing nosocomial pathogens shifting their phenotypes to that of pro-inflammatory and lethal strains.1 This highly evolved “sense and respond” circuitry in certain opportunistic organisms allows them to sense host vulnerability and respond accordingly.1
We have been interested in the mechanism by which the human opportunistic pathogen Pseudomonas aeruginosa, a major cause of infectious-related mortality in critically ill patients, is activated to express a virulent phenotype within the intestinal tract of a surgically stressed host. We have developed a unique model of gut-derived sepsis whereby P. aeruginosa is introduced directly into the cecum of mice surgically stressed with a 30% hepatectomy resulting in lethal gut-derived sepsis.2 Mortality in this model is not due to bacterial dissemination from the gut but rather to the ability of P. aeruginosa to alter the intestinal epithelial barrier to its highly cytotoxic exoproducts. In this regard, we have shown that the PA-I lectin of P. aeruginosa, a binding adhesin and potent intestinal epithelial barrier dysregulating protein, is in vivo expressed in the intestine of mice following 30% hepatectomy and directly alters the tight junctional permeability of the intestinal epithelium to exotoxin A resulting in lethal gut derived sepsis.3 Multiple soluble host factors including interferon-gamma, the endogenous opiate dynorphin, and the hypoxic end-products adenosine and inosine2,4,5 are released into the intestinal lumen that directly bind to and activate the virulence of P. aeruginosa to express PA-I and other virulence factors.
Among the various local intestinal environmental cues that might activate the virulence of P. aeruginosa to shift its phenotype to that of a lethal strain during acute surgical injury is the local intestinal concentration of phosphate. P. aeruginosa virulence gene activation is highly sensitive to depletion of extracellular phosphate which is known to occur following acute surgical injury.6 P. aeruginosa senses low extracellular phosphate via phosphate sensors located within and on its outer membrane.7 We have previously shown that a phosphate scavenging protein in P. aeruginosa termed PstS, is expressed on outer appendages during acute phosphate depletion and mediates a potent barrier disrupting response against the intestinal epithelium.8 These effects are mediated via the well described quorum sensing signaling system (QS) in P. aeruginosa which is a hierarchical system of virulence gene regulation controlling the expression of multiple virulence products in P. aeruginosa.9,10
Since severe hypophosphatemia has been observed to precipitously develop following major surgical injury and is an independent predictor of mortality during sepsis11, we explored the possibility that opportunistic pathogens directly sense low phosphate conditions within the gut during surgical injury as a signal of host injury and activate their virulence circuitry to cause lethal gut-derived sepsis. Therefore the aims of this study were: 1.) to determine if phosphate depletion activates key virulence factors in P. aeruginosa known to be involved in intestinal epithelial barrier disruption and gut-derived sepsis such as the expression of the PA-1 lectin, biofilm formation, pyocyanin production, and PstS expression; 2.) to determine whether local concentrations of phosphate become depleted following surgical injury (30% hepatectomy); and 3.) to determine if phosphate repletion will protect mice from gut-derived sepsis due to P. aeruginosa.
Materials and Methods
In vitro virulence assays
Experimental Protocol
In order to determine if phosphate depletion enhances the virulence of P. aeruginosa in vitro, we assayed multiple virulence factors known to be associated with altered intestinal epithelial barrier function and lethal gut-derived sepsis including the PA-I lectin, a known intestinal epithelial barrier disrupting protein; pyocyanin, a redox active pigment that causes epithelial cell oxidative stress and induces neutrophil apoptosis; biofilm, a bacterial slime that allows bacteria to persist on epithelial surfaces and resist antibiotics as well as immune elimination, and PstS, a phosphate acquisition protein that is activated under conditions of low phosphate to scavenge extracellular phosphate. Phosphate depletion was created by growing P. aeruginosa in phosphate rich media (15 mM) to an exponential state (OD=0.7) over a period of five hours, then removing the media by centrifugation and immediately replacing it with low phosphate media (0.1 mM). Cultures were allowed to grow for 24 hours, samples were then collected and centrifuged and their supernatants and pellets collected and placed at −80°C for further analysis. Phosphate sufficient and deficient media were designed based on modified media described by Hancock.12 Briefly, all media contained the following autoclaved components: 0.5mM MgSO4, 0.1M HEPES pH 7.0, 7mM (NH4)2SO4, 20mM disodium succinate, trace ion mixture containing 0.1% of 2.45 mM CaCl2, 13.91 mM ZnCl2, 4.69mM H3BO4, and 0.67mM CoCl2, 1.78mM FeSO4. Phosphate sufficient media was supplemented with a stock solution of KH2PO4 for a final concentration of 15 mM KH2PO4 whereas phosphate deficient media contained 0.1 mM KH2PO4. All media were standardized to a pH of 7.4.
Bacterial Strains
P. aeruginosa strains MPAO1 and SPAO1 and their derivatives were routinely grown overnight prior to all experiments in tryptic soy broth (TSB), a phosphate-rich media containing 45mM phosphate. P. aeruginosa wild-type strain MPAO1 wild-type was obtained from the P. aeruginosa mutant library.13 P. aeruginosa strain SPAO1 and its derivative mutant ΔlecA (PA-1 lectin) were obtained from Stephen B. Diggle.
PA-1 lectin and PstS
PA-I lectin and PstS protein were assayed using standard ELISA techniques with specific antibody as previously described.5,14 Assays were normalized to total protein content in samples using BCA protein assay (Thermo Scientific). All experiments were performed in triplicate.
Biofilm Assay
Biofilm formation was assayed as described with modifications.15 P. aeruginosa was grown in 96-well plates containing varying concentrations of phosphate. Plates were incubated at 37°C under mild shaking at 50 rpm for 24 hours. The wells were then rinsed thoroughly with water and the attached material was stained with 0.1% crystal violet, washed with water, and solubilized in ethanol. Solubilized fractions were collected and absorbance measured at 550 nm with a plate reader. Bacterial suspensions containing high phosphate (15 mM), low phosphate (0.1 mM) as well as phosphate solutions containing no bacteria were also analyzed. All experiments were performed in triplicate.
Pyocyanin Assay
Pyocyanin formation was assayed as described by Zaborina et al.5 Briefly, following 24-hour incubation in varying concentrations of phosphate, bacterial cultures were spun down by centrifugation and 1 mL of supernatant was extracted using 500 µL of chloroform, then re-extracted with 150 µL of 0.2 M HCl. Absorbance was measured at OD 520 nm. Appropriate controls were analyzed including bacterial suspensions containing high phosphate (15 mM), low phosphate (0.1 mM) as well as phosphate solutions containing no bacteria. All experiments were performed in triplicate.
Mouse Model of Lethal Gut-derived Sepsis
Experimental Protocol
All experiments were approved by the Animal Care and Use Committee at the University of Chicago (IACUC protocol 71744). Male C57BL6/HSD mice weighing 18g–22 g were used for all experiments. Mice were routinely fed tap water and Harland Teklad feed. To determine whether surgical injury may affect serum and/or local intestinal phosphate concentrations, samples were collected prior to and 24 hours following a 30% hepatectomy and intestinal inoculation with P. aeruginosa into the cecum as previously described.2 A volume of 200 µL (~107 CFU), containing P. aeruginosa MPAO1 suspended in 10% glycerol was introduced into the cecum via direct puncture following a bloodless 30% hepatectomy. Animals were allowed water ad libitum only for the remainder of the study period. While intestinal microbiota are present within the fecal stream, bacterial densities are greatest along the adherent mucus layer lining the intestinal epithelium.16 Additionally, the majority of intestinal phosphate absorption in mice occurs in the ileum.17 Therefore samples from both the intestinal filtrate (fecal stream) and intestinal mucus layer were collected from the distal ileum and analyzed separately for phosphate measurement 24 hours following 30% hepatectomy.
Four Groups of mice were studied. 1) normal chow diet; 2) 24-hour fast consisting of tap water only; 3) 24-hour fast consisting of tap water followed by 30% hepatectomy, and 4) 24-hour fast, 30% hepatectomy along with ileocecal injection of P. aeruginosa strain MPAO1. Groups of five mice were sacrificed 24 hours post-operatively for collection of blood and intestinal samples to be analyzed for phosphate content. Serum phosphate samples were obtained by puncture of the retrobulbar venous plexus using a Pasteur pipette and deposited into plasma separator tubes. A constant 10 cm segment of the distal ileum was collected just proximal to the cecum. Harvest of both intestinal luminal contents and the mucus layer were performed similarly as described by Katayama et al.18 Briefly, one mL of cold 0.9 normal saline (NS) was injected into each segment and the luminal contents (including stool) were collected. Afterward, each intestinal segment was opened sharply in a longitudinal fashion, the intestinal mucus layer was carefully removed with a fine glass cover slip and placed into 1mL cold 0.9 NS. All samples were homogenized, centrifuged, and their supernatants analyzed for phosphate content.
Measurement of Inorganic Phosphate
All samples were assayed for the quantitative determination of phosphorus using a Roche automated clinical chemistry analyzer at the University of Chicago Hospitals Medical Laboratories.14
Phosphate repletion experiments
As this model of lethal gut-derived sepsis is known to result in 60% mortality at 48 hrs, we wished to determine whether phosphate repletion could improve survival. Phosphate was repleted in groups of mice (n=10) both systemically and locally using a solution of phosphate-buffered saline (PBS) prepared by mixing 0.23 g NaH2PO4, 1.15 g Na2HPO4, and 8.75 g NaCl to 1 L of Milli-Q water and pH adjusted with 1M NaOH to 7.4. Systemic repletion (IV) was accomplished by injecting 0.3 mL of 5 mM PBS into the retrobulbar venous plexus one hour prior to and 6 hours following surgery. Local intestinal repletion was accomplished by supplying drinking water consisting of tap water supplemented with PBS (final concentration = 10 mM phosphate) for a 24 hr period prior to and for 48 hours following hepatectomy. Additionally, mice in the local repletion group were inoculated with intestinal P. aeruginosa dissolved in a solution of phosphate-buffered saline (PBS) supplemented with 10 mM phosphate. Since dietary phosphate supplementation may bolster immune function in mice, reiterative studies (n = 10) were performed in which mice were inoculated with intestinal P. aeruginosa dissolved in a solution of PBS supplemented with 10 mM phosphate only without dietary phosphate supplementation. Mice in all control groups received equivalent repletion solutions substituted with normal saline. Groups were followed for both 48-hour mortality and separately for 24-hour serum and intestinal phosphate collection, as described above.
In vivo expression of PstS in P. aeruginosa
To determine whether P. aeruginosa can “sense” local intestinal phosphate concentrations in vivo, P. aeruginosa strain MPAO1 was injected into the cecum of mice (n=3) with and without local phosphate repletion following 30% hepatectomy and then retrieved from the distal intestinal tract 24 hours later. Since intestinal phosphate concentrations may differ between the luminal filtrate and mucus layer under conditions of surgical stress, P. aeruginosa was retrieved from each “compartment” and analyzed separately. Collected bacterial RNA was used to determine relative in vivo expression of PstS, a phosphate scavenging protein by standard real-time reverse transcription (RT)-PCR techniques.5
Statistical Analysis
All statistical analysis of the data was performed using Student’s t-test and analysis of variance (ANOVA) with Microsoft Excel and Sigma Plot software. Kaplan-Meier Survival analysis was performed using SPSS 15.0 software.
Results
Phosphate depletion results in increased PA-1 lectin expression, pyocyanin production, biofilm formation, and PstS expression in vitro
Results are summarized in Figure 1. Results demonstrate that phosphate depletion increased the virulence of P. aeruginosa as judged by increase in PA-1 lectin, pyocanin production, biofilm formation, and PstS expression.
Figure 1.
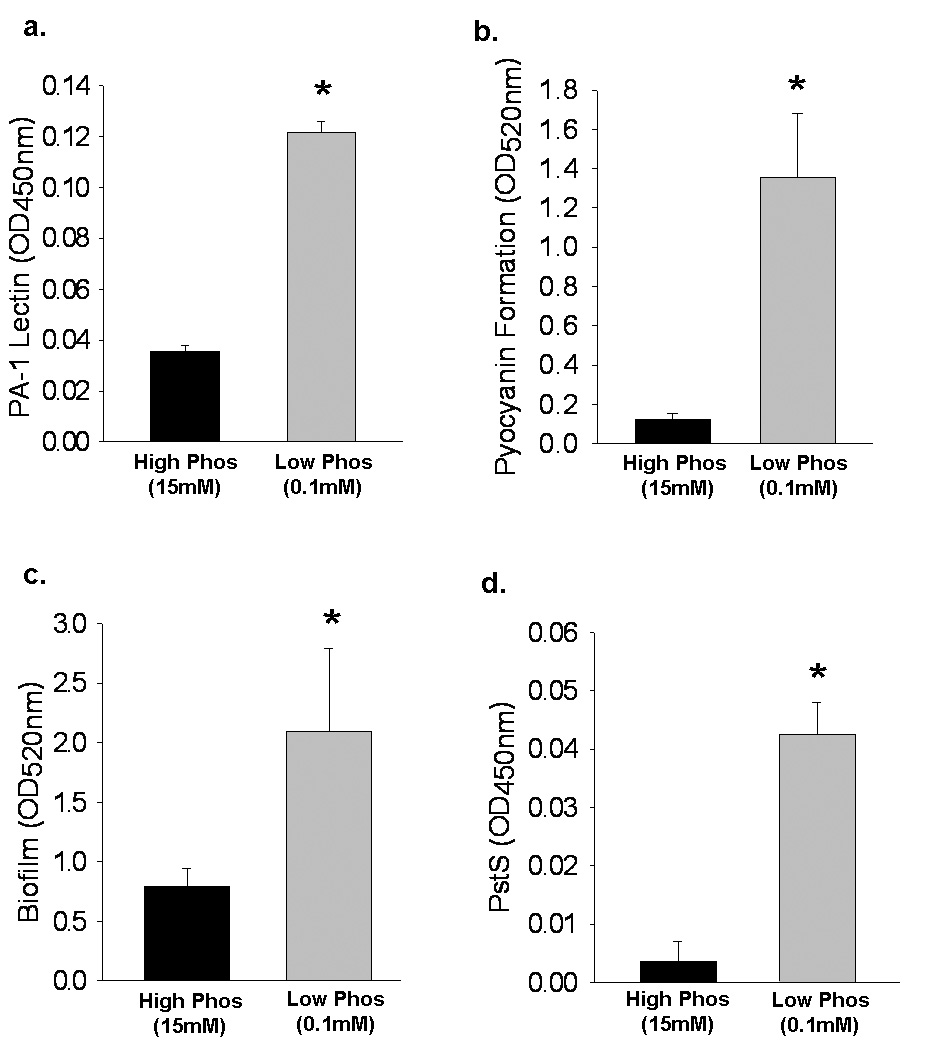
Effect of phosphate depletion on P. aeruginosa virulence expression in vitro. Cultures of P. aeruginosa were grown in high phosphate media (15 mM) until log-phase growth and then transferred to either high phosphate media (15 mM) or low phosphate media (0.1 mM) and allowed to grow for an additional 24 hours. For all experiments, n=9 observations per group. Results are mean ± SD for all groups. (A) PA-1 lectin expression, as measured by ELISA; * p=.001. (B) PCN production as measured by absorbance (OD520nm) following chloroform and HCl extraction of culture supernatants; * p=.002. (C) Biofilm formation as measured by absorbance (OD550nm); * p=.01; (D) PstS expression as measured by ELISA; * p<.001.
30% Hepatectomy in mice results in significantly depleted phosphate concentration within the intestinal mucus layer
Results are summarized in Figure 2. Results demonstrate the relationship between phosphate content present in serum, the intestinal mucus layer, and the intestinal luminal filtrate under increasing degrees of catabolic stress. Whereas phosphate content was progressively depleted in the intestinal mucus layer with increasing stress, there was no appreciable change in the luminal filtrate phosphate content or serum phosphate. Within the intestinal mucus layer, a 30% surgical hepatectomy resulted in a critically low phosphate concentration sufficient for activation of P. aeruginosa virulence phenotypes in vitro (i.e. < 0.1 mM phosphate), an effect not seen within the luminal filtrate nor in the serum.
Figure 2.
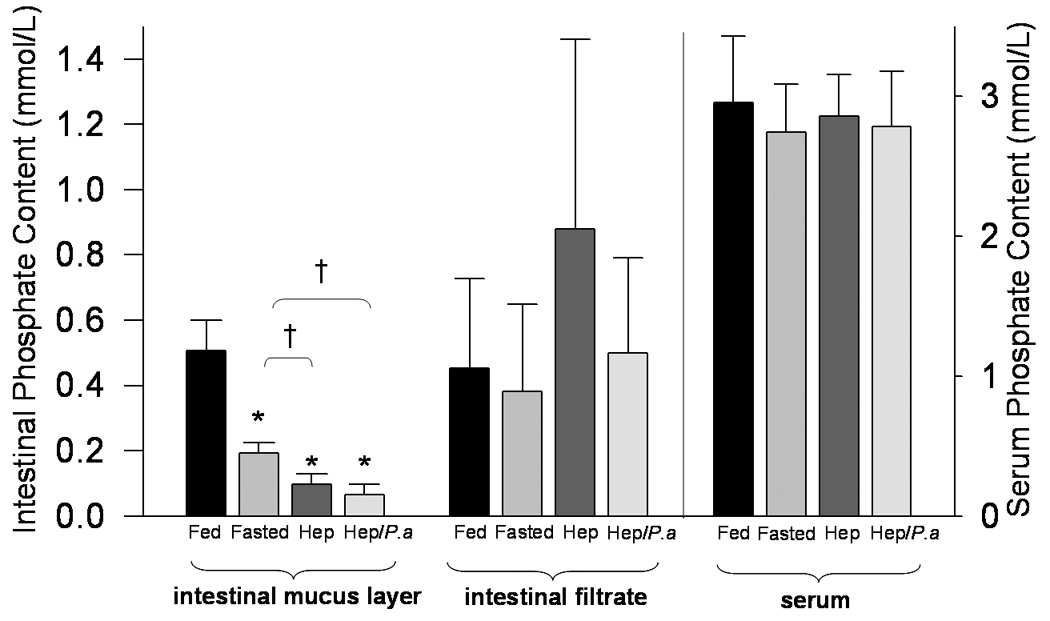
Effect of surgical stress on gastrointestinal and serum phosphate content in mice. Groups of mice (n=5) were divided into fed controls (Fed), fasting controls (Fasted), fasting + hepatectomy (Hep), or fasting + hepatectomy + intestinal inoculation of P. aeruginosa (Hep/P.a). Mice were sacrificed 24 hours following surgery and the intestinal luminal contents (intestinal filtrate), the intestinal mucus layer, and serum were collected separately and phosphate content measured (mmol/L). The left y-axis denotes intestinal phosphate content (mmol/L) and the right y-axis denotes serum phosphate content (mmol/L); * p<.001, † p=.005; results are mean ± SD for all groups.
Dietary phosphate repletion in mice increased intestinal phosphate content and prevented mortality
Results are summarized in Figure 3. A 30% hepatectomy + injection of P. aeruginosa into the cecum resulted in high mortality (60%) that was PA-I dependent since the mutant strain Δ PA-I lacking PA-I lectin failed to induce mortality in this model (Figure 3a). Oral phosphate repletion significantly increased the intestinal mucus layer phosphate content and protected mice against mortality while systemic administration (IV) neither restored mucus layer phosphate nor protected against mortality (Figure 3b & 3c).
Figure 3.

(A) PA-1 lectin is required for P. aeruginosa to cause lethal gut-derived sepsis. 48-hour Kaplan-Meier survival analysis of groups of mice (n=10) following 30% surgical hepatectomy and intestinal inoculation with either wild-type P. aeruginosa (Hep + P.a) or its complimentary PA-1 lectin mutant, ΔlecA (Hep + P.a ΔlecA), p=.005. (B) Effect of oral versus systemic (IV) phosphate (Pi) repletion on mortality in gut-derived sepsis due to P. aeruginosa. 48-hour Kaplan-Meier survival analysis of groups of mice (n=10) following 30% surgical hepatectomy and intestinal inoculation with P. aeruginosa (Hep/P.a); 30% surgical hepatectomy in mice given oral Pi repletion along with intestinal inoculation of P. aeruginosa suspended in Pi (Hep/P.a + oral Pi); 30% surgical hepatectomy and intestinal inoculation of P. aeruginosa suspended in Pi only (Hep/P.a + Pi susp); 30% surgical hepatectomy in mice given IV Pi and intestinal inoculation of P. aeruginosa (Hep/P.a. + IV Pi); p=.025 Hep/P.a + oral Pi vs. Hep/P.a. (C) Effect of oral and systemic (IV) phosphate repletion on the intestinal mucus layer of mice. Groups of mice (n=5) underwent 30% surgical hepatectomy and intestinal inoculation of P. aeruginosa alone (Hep/P.a), 30% surgical hepatectomy in mice given IV Pi and intestinal inoculation of P. aeruginosa (Hep/P.a + IV Pi), or 30% surgical hepatectomy in mice given oral Pi repletion + intestinal inoculation of P. aeruginosa suspended in Pi (Hep/P.a + oral Pi); * p<.001, results are mean ± SD for all groups.
Intestinal P. aeruginosa senses surgically-induced low intestinal phosphate in vivo
Results are summarized in Figure 4 and demonstrate increased in vivo expression of P. aeruginosa PstS as measured by RT-PCR within the mucus layer of fasted mice having undergone hepatectomy, an effect not seen in intestinal filtrates (Figure 4b). Oral phosphate repletion attenuated P. aeruginosa PstS expression in mice following hepatectomy to a level equivalent to that seen in non-operated controls. Fasting and surgical hepatectomy also resulted in a significantly higher level of citrate synthetase expression, a constitutive P. aeruginosa housekeeping gene, within the intestinal mucus layer as compared to the intestinal filtrate (Figure 4a).
Figure 4.

Expression of citrate synthetase and PstS in mucus versus intestinal filtrate in mice following 30% hepatectomy. Groups of mice (n=6 observations/group) were assigned to 1.) fed controls (control), 2.) fasted controls (Fasted), 3.) 30% surgical hepatectomy and intestinal inoculation of P. aeruginosa (Hep/P.a), and 4.) 30% surgical hepatectomy + oral Pi repletion and intestinal inoculation of P. aeruginosa suspended in Pi (Hep/P.a + oral Pi). (A) To determine the relative density of P. aeruginosa in intestinal filtrates versus mucus between groups, constitutively expressed citrate synthetase was measured using RT-PCR; * p<.001, † p<.001. (B) Similarly, to determine the relative concentration of Pi in intestinal filtrates versus intestinal mucus between groups, PstS expression, induced only under low Pi conditions, was measured by RT-PCR; * p<.001, † p<.001.
Discussion
Data from the present study demonstrate that a major environmental cue to which intestinal P. aeruginosa responds with enhanced virulence is local depletion of phosphate. The association of serum hypophosphatemia and sepsis is well established and is especially suggestive of gram-negative infections19,20. Zazzo et al. reported that mortality was increased among surgical intensive care patients with hypophosphatemia versus those with a normal serum Pi (30% vs 15.2%, respectively; p <0.05).21 Likewise, Shor et al examined the charts of 6,190 septic patients and stratified them into 2 groups: group 1 comprised 26 patients with severe hypophosphatemia (serum Pi < 1 mg/dL); group 2 comprised 29 patients without severe hypophosphatemia (serum Pi > 1mg/dL). Severe hypophosphatemia was found to independently predict mortality, conferring a nearly 8-fold risk of death (odds ratio = 7.98, 95% CI = 2.3 – 27.6). A precipitous decrease in serum phosphate has also been reported to develop in response to major liver resection and this could likewise predispose to infectious-related mortality, however the precise mechanism of this response remains largely unknown.6 Data from the present study demonstrate not only that phosphate depletion in vitro activates virulence in P. aeruginosa in vitro, but that P. aeruginosa “senses” low phosphate conditions in vivo based on our finding of increased PstS expression in P. aeruginosa RNA harvested from the intestinal tract of mice following hepatectomy. The finding of a near 32-fold increase in PstS expression, suggests that P. aeruginosa sensed a relatively inadequate concentration of phosphate when present in the intestinal mucus layer of mice following a surgical hepatectomy. This finding is consistent with recent fluorescence in-situ hybridization studies (FISH) that confirm that the greatest density of intestinal bacteria reside on and within the intestinal mucus layer where they consume the majority of their nutrients.22 That PstS in P. aeruginosa was in vivo expressed in the intestine during surgical injury is intriguing and is molecularly linked to activation of additional quorum sensing dependent virulence factors including the intestinal barrier dysregulating proteins (PA-I), compounds to disarm neutrophils (pyocyanin), and exoproducts that protect against antibiotics and immune cells (biofilm production). The proposed link between phosphoregulatory pathways and quorum sensing signaling system in P. aeruginosa further emphasize the importance of the observations in the present study.9
Virtually all bacteria possess systems of phosphate sensing and acquisition, although they are activated at different thresholds of Pi concentration. Interestingly P. aeruginosa, an accidental pathogen to man, activates its phosphoregulatory and virulence pathways at Pi concentrations that are much higher compared to other bacteria such as E. coli23 possibly accounting for the observation that its mere presence in the intestinal tract of a critically ill patient confers a 3-fold increase in mortality.24 P. aeruginosa is present in the feces of up to 50% of critically ill patients and carries the highest case fatality rate of any nosocomial pathogen.25 The ability of P. aeruginosa to form a protective biofilm and induce lethality without the need to disseminate from the gut makes its presence in this site highly problematic. Yet its presence may be less important than its in vivo activation. Findings in the present study that local but not systemic phosphate repletion was completely protective may be clinically relevant to current methods to replete phosphate in critically ill patients. Further work in our laboratory has demonstrated that under conditions of high extracellular phosphate, P. aeruginosa becomes relatively insensate to host compounds known to activate its virulence (unpublished observations). Results from the present study suggest that a possible approach to prevent feared pathogens such as P. aeruginosa that colonize the gastrointestinal tract from causing sepsis, may be to maintain high local levels of intestinal phosphate. There are no studies in humans that have determined the concentrations of phosphate within the in the distal intestinal tract mucus during surgical stress. Yet, based on the available information on the dynamics of phosphate redistribution during surgical injury, it is very unlikely that systemic (IV) phosphate loading will replenish locally depleted phosphate in the intestine as the phosphatonin hormones, elevated in response to surgical injury, drive excess phosphaturia, intracellular phosphate shifts, and phosphate depletion.6,26 In addition the intestinal mucus consists of a layer of mucin lined by a film of phospholipids which themselves become rapidly depleted during surgical injury and gut starvation.27–29 While the commensal microflora may not respond to this local depletion without virulence expression as their activation potentials respond at much lower concentrations of Pi, intestinal colonization by nosocomial pathogens during critical illness may represent a microbial consortia much more activated by local Pi conditions, and hence much more virulent.
Intriguingly, the concentration of serum phosphate that correlates with sepsis induced mortality in critically ill patients, <0.3–0.4 mmol/L11, is the same in vitro concentration that results in P. aeruginosa chemotaxis, expression of phosphate scavenging proteins, and activation of virulence genes via quorum sensing.9,30,31 Data from the present study show for the first time to our knowledge that acute phosphate depletion occurs within the intestinal mucus layer of the distal ileum following 30% hepatectomy. In this study, mice that were fasted and underwent hepatectomy with intestinal inoculation of P. aeruginosa had significantly lower intestinal mucus layer phosphate content versus mice that were fasted only (0.06 ± .03 vs. 0.19 ± .03 mmol/L, p = .008). This finding is important since acute depletion of intestinal phosphate concentrations from 0.51 ± .09 mmol/L in fed mice to 0.06 ± .03 mmol/L in mice that are allowed water only and undergo partial hepatectomy is within the threshold at which P. aeruginosa is activated to scavenge for phosphate sources and increase expression of various virulence factors such as phosphatases that break down organic phosphates.30 Mice that were allowed water only had significantly lower intestinal mucus phosphate concentrations than fed mice, suggesting that starvation plays a significant role in the observed depletion of Pi. We suspect the additional depletion seen with partial hepatectomy is due to phosphatonin driven phosphaturia.26 As total body phosphorus is depleted in the mouse due to renal losses, net intestinal absorption is maximized thus depleting available phosphorus sources within the distal intestinal mucus layer (Figure 5). Taken together, these data beg a more complete understanding of not only the actual concentration of Pi present in the intestinal mucus of the distal intestinal tract during critical illness but also of the virulence pathways that problematic pathogens activate to acquire Pi. Elucidation of these subtle mechanisms might explain why culture negative gut-derived sepsis continues to remain clinically elusive and unresponsive to more powerful and escalating doses of antibiotics.
Figure 5.
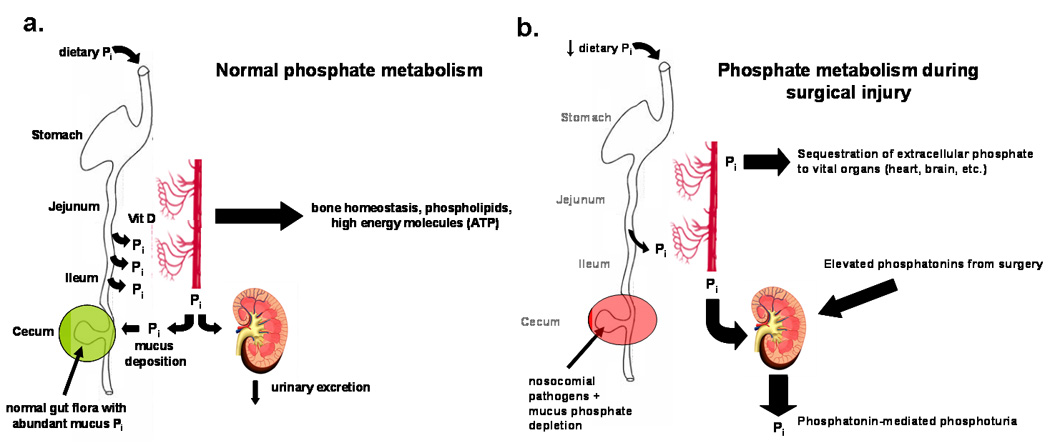
Proposed redistribution of extracellular Pi via phosphatonin release during surgical injury. (A) While in humans dietary Pi is absorbed proximally in the jejunum, in mice dietary Pi absorption occurs in the ileum and is regulated by Vitamin D. Urinary Pi excretion remains low and intestinal mucus Pi is abundant. (B) Surgical injury depletes extracellular Pi via phosphatonin induced phosphaturia resulting in low Pi in intestinal mucus in mice and low serum Pi in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alverdy J, Holbrook C, Rocha F, Seiden L, Wu RL, Musch M, Chang E, Ohman D, Suh S. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann Surg. 2000;232(4):480–489. doi: 10.1097/00000658-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu LR, Zaborina O, Zaborin A, Chang EB, Musch M, Holbrook C, Turner JR, Alverdy JC. Surgical injury and metabolic stress enhance the virulence of the human opportunistic pathogen Pseudomonas aeruginosa. Surg Infect (Larchmt) 2005;6(2):185–195. doi: 10.1089/sur.2005.6.185. [DOI] [PubMed] [Google Scholar]
- 3.Wu L, Holbrook C, Zaborina O, Ploplys E, Rocha F, Pelham D, Chang E, Musch M, Alverdy J. Pseudomonas aeruginosa expresses a lethal virulence determinant, the PA-I lectin/adhesin, in the intestinal tract of a stressed host: the role of epithelia cell contact and molecules of the Quorum Sensing Signaling System. Ann Surg. 2003;238(5):754–764. doi: 10.1097/01.sla.0000094551.88143.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu L, Estrada O, Zaborina O, Bains M, Shen L, Kohler JE, Patel N, Musch MW, Chang EB, Fu YX, Jacobs MA, Nishimura MI, Hancock RE, Turner JR, Alverdy JC. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309(5735):774–777. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- 5.Zaborina O, Lepine F, Xiao G, Valuckaite V, Chen Y, Li T, Ciancio M, Zaborin A, Petroff E, Turner JR, Rahme LG, Chang E, Alverdy JC. Dynorphin activates quorum sensing quinolone signaling in Pseudomonas aeruginosa. PLoS Pathog. 2007;3(3):e35. doi: 10.1371/journal.ppat.0030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta HK, Malik M, Neely RD. Hepatic surgery-related hypophosphatemia. Clin Chim Acta. 2007;380(1–2):13–23. doi: 10.1016/j.cca.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Tam R, Saier MH., Jr Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57(2):320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaborina O, Holbrook C, Chen Y, Long J, Zaborin A, Morozova I, Fernandez H, Wang Y, Turner JR, Alverdy JC. Structure-Function Aspects of PstS in Multi-Drug-Resistant Pseudomonas aeruginosa. PLoS Pathog. 2008;4(2):e43. doi: 10.1371/journal.ppat.0040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen V, Lons D, Zaoui C, Bredenbruch F, Meissner A, Dieterich G, Munch R, Haussler S. RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and -independent pathways. J Bacteriol. 2006;188(124):8601–8606. doi: 10.1128/JB.01378-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311(5764):1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shor R, Halabe A, Rishver S, Tilis Y, Matas Z, Fux A, Boaz M, Weinstein J. Severe hypophosphatemia in sepsis as a mortality predictor. Ann Clin Lab Sci. 2006;36(1):67–72. [PubMed] [Google Scholar]
- 12.Hancock RE, Raffle VJ, Nicas TI. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981;19(5):777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2003;100(24):14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaborina O. Structure-Function Aspects of PstS in Multi-Drug Resistant Pseudomonas aeruginosa. PLoS Pathog. 2008 doi: 10.1371/journal.ppat.0040043. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaborina O, Kohler JE, Wang Y, Bethel C, Shevchenko O, Wu L, Turner JR, Alverdy JC. Identification of multi-drug resistant Pseudomonas aeruginosa clinical isolates that are highly disruptive to the intestinal epithelial barrier. Ann Clin Microbiol Antimicrob. 2006;5:14. doi: 10.1186/1476-0711-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnenburg JL, Angenent LT, Gordon JI. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat Immunol. 2004;5(6):569–573. doi: 10.1038/ni1079. [DOI] [PubMed] [Google Scholar]
- 17.Marks J, Srai SK, Biber J, Murer H, Unwin RJ, Debnam ES. Intestinal phosphate absorption and the effect of vitamin D: a comparison of rats with mice. Exp Physiol. 2006;91(3):531–537. doi: 10.1113/expphysiol.2005.032516. [DOI] [PubMed] [Google Scholar]
- 18.Katayama M, Xu D, Specian RD, Deitch EA. Role of bacterial adherence and the mucus barrier on bacterial translocation: effects of protein malnutrition and endotoxin in rats. Ann Surg. 1997;225(3):317–326. doi: 10.1097/00000658-199703000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riedler GF, Scheitlin WA. Hypophosphataemia in septicaemia: higher incidence in gram-negative than in gram-positive infections. Br Med J. 1969;1(5646):753–756. doi: 10.1136/bmj.1.5646.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Landenberg P, Shoenfeld Y. New approaches in the diagnosis of sepsis. Isr Med Assoc J. 2001;3(6):439–442. [PubMed] [Google Scholar]
- 21.Zazzo JF, Troche G, Ruel P, Maintenant J. High incidence of hypophosphatemia in surgical intensive care patients: efficacy of phosphorus therapy on myocardial function. Intensive Care Med. 1995;21(10):826–831. doi: 10.1007/BF01700966. [DOI] [PubMed] [Google Scholar]
- 22.Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dorffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56(3):343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Dien SJ, Keasling JD. A dynamic model of the Escherichia coli phosphate-starvation response. J Theor Biol. 1998;190(1):37–49. doi: 10.1006/jtbi.1997.0524. [DOI] [PubMed] [Google Scholar]
- 24.Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The "undrained abscess" of multiple organ failure. Ann Surg. 1993;218(2):111–119. doi: 10.1097/00000658-199308000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertrand X, Thouverez M, Talon D, Boillot A, Capellier G, Floriot C, Helias JP. Endemicity, molecular diversity and colonisation routes of Pseudomonas aeruginosa in intensive care units. Intensive Care Med. 2001;27(8):1263–1268. doi: 10.1007/s001340100979. [DOI] [PubMed] [Google Scholar]
- 26.Salem RR, Tray K. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2005;241(2):343–348. doi: 10.1097/01.sla.0000152093.43468.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Vilar J, Mabolo R. Gel-forming mucins. Notions from in vitro studies. Histol Histopathol. 2007;22(4):455–464. doi: 10.14670/HH-22.455. [DOI] [PubMed] [Google Scholar]
- 29.Okuyama H, Urao M, Lee D, Abe A, Drongowski RA, Harmon CM, Coran AG. Changes, with age, in the phospholipid content of the intestinal mucus layer of the newborn rabbit. J Pediatr Surg. 1998;33(1):35–38. doi: 10.1016/s0022-3468(98)90356-6. [DOI] [PubMed] [Google Scholar]
- 30.Hancock RE, Poole K, Benz R. Outer membrane protein P of Pseudomonas aeruginosa: regulation by phosphate deficiency and formation of small anion-specific channels in lipid bilayer membranes. J Bacteriol. 1982;150(2):730–738. doi: 10.1128/jb.150.2.730-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato J, Ito A, Nikata T, Ohtake H. Phosphate taxis in Pseudomonas aeruginosa. J Bacteriol. 1992;174(15):5149–5151. doi: 10.1128/jb.174.15.5149-5151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


