Abstract
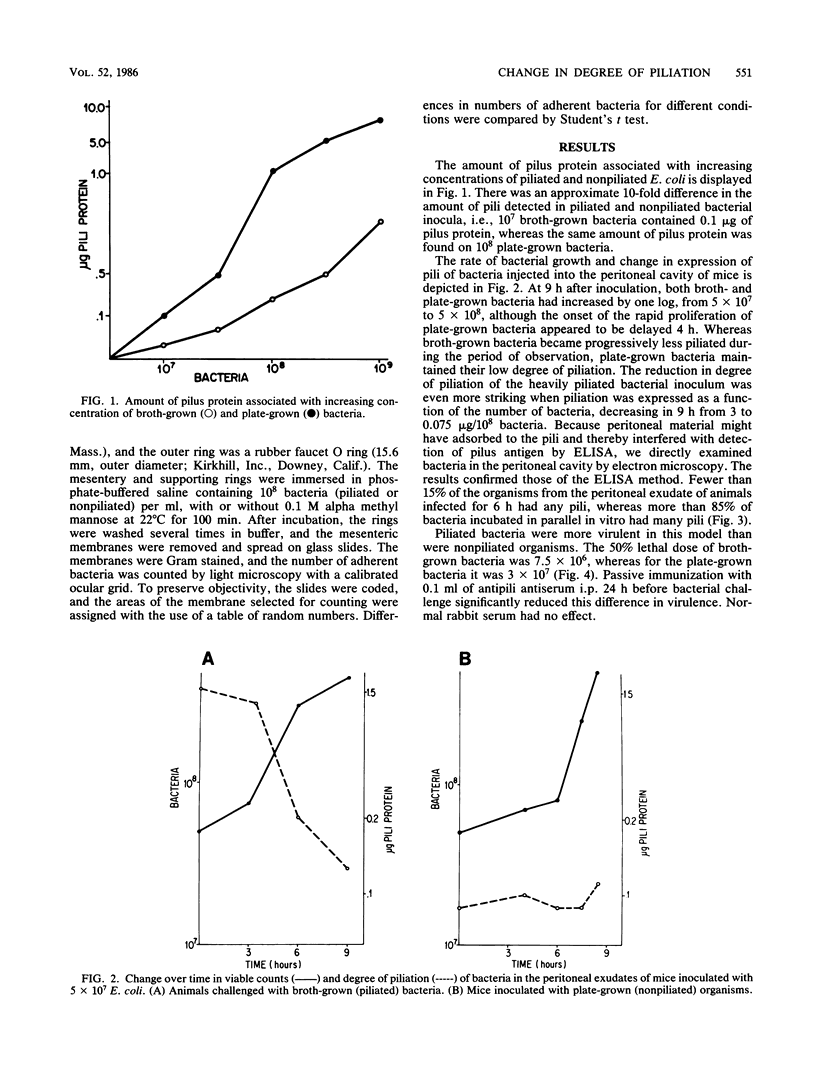
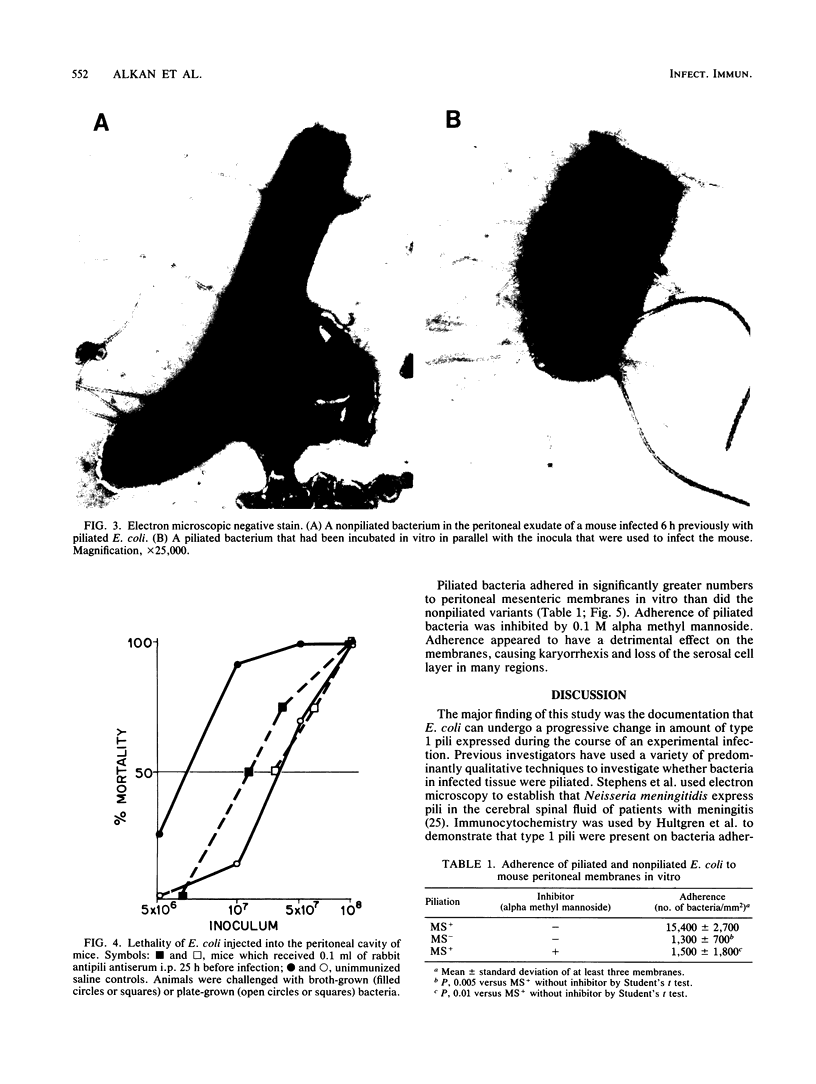
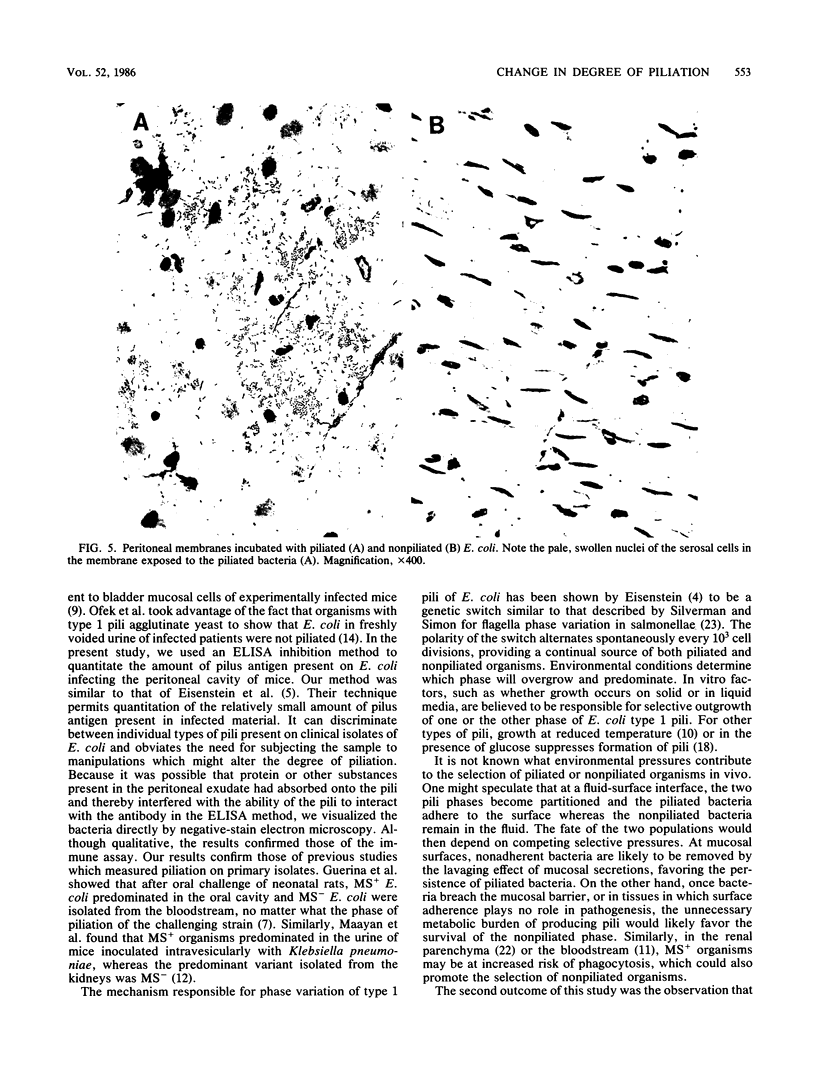
To determine whether expression of type 1 pili varies during the course of Escherichia coli infection in vivo, mice were injected intraperitoneally with 5 X 10(7) CFU of piliated or nonpiliated phase variants per ml, and the degree of piliation was measured in peritoneal exudate by an enzyme-linked immunosorbent assay inhibition method. In the animals challenged with the piliated bacteria, the numbers of organisms increased a log over 9 h and the amount of pilus antigen decreased from 3 to 0.075 micrograms/10 bacteria. After a 4-h delay, nonpiliated bacteria also increased by one log over 9 h; however, the amount of piliation remained virtually undetectable. Piliated E. coli were more virulent than nonpiliated variants in this model (50% lethal dose of 7.5 X 10(6) versus 3 X 10(7), respectively). The difference was significantly reduced by prior passive immunization with rabbit serum containing high titers of antipili antibody. Piliated bacteria adhered in significantly greater numbers to isolated mouse peritoneal membranes than did nonpiliated variants (15,400 +/- 2,700 versus 1,300 +/- 700 bacteria/mm2, respectively; P = 0.05). Adherence was inhibited by the presence of 0.1 M alpha methyl mannose (1,500 +/- 1,800 bacteria/mm2, P = 0.01). These results confirm the results of previous qualitative studies showing that phase variation of type 1 pili occurs in vivo and suggest that these pili may confer an initial advantage for growth of E. coli in the peritoneal cavity, presumably by fostering colonization of the peritoneal serosal surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Clements J. R., Dodd D. C. Isolation and characterization of a monoclonal antibody directed against type 1 fimbriae organelles from Escherichia coli. Infect Immun. 1983 Oct;42(1):333–340. doi: 10.1128/iai.42.1.333-340.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Silverblatt F. J., Sharon N. Dissociation and reassembly of Escherichia coli type 1 pili. J Bacteriol. 1981 Oct;148(1):308–314. doi: 10.1128/jb.148.1.308-314.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerina N. G., Kessler T. W., Guerina V. J., Neutra M. R., Clegg H. W., Langermann S., Scannapieco F. A., Goldmann D. A. The role of pili and capsule in the pathogenesis of neonatal infection with Escherichia coli K1. J Infect Dis. 1983 Sep;148(3):395–405. doi: 10.1093/infdis/148.3.395. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Porter T. N., Schaeffer A. J., Duncan J. L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun. 1985 Nov;50(2):370–377. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Leunk R. D., Moon R. J. Association of type 1 pili with the ability of livers to clear Salmonella typhimurium. Infect Immun. 1982 Jun;36(3):1168–1174. doi: 10.1128/iai.36.3.1168-1174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan M. C., Ofek I., Medalia O., Aronson M. Population shift in mannose-specific fimbriated phase of Klebsiella pneumoniae during experimental urinary tract infection in mice. Infect Immun. 1985 Sep;49(3):785–789. doi: 10.1128/iai.49.3.785-789.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mosek A., Sharon N. Mannose-specific adherence of Escherichia coli freshly excreted in the urine of patients with urinary tract infections, and of isolates subcultured from the infected urine. Infect Immun. 1981 Dec;34(3):708–711. doi: 10.1128/iai.34.3.708-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Duguid J. P. Selective outgrowth of fimbriate bacteria in static liquid medium. J Bacteriol. 1970 Aug;103(2):447–456. doi: 10.1128/jb.103.2.447-456.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Weinstein W. M., Sullivan N. M., Bartlett J. G., Gorbach S. L. Experimental intra-abdominal abscesses in rats: quantitative bacteriology of infected animals. Infect Immun. 1974 Dec;10(6):1256–1259. doi: 10.1128/iai.10.6.1256-1259.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Schmidt M. R., Leibowitz M. Cyclic AMP-dependent synthesis of fimbriae in Salmonella typhimurium: effects of cya and pts mutations. J Bacteriol. 1978 Apr;134(1):356–358. doi: 10.1128/jb.134.1.356-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Influence of pili on the virulence of Proteus mirabilis in experimental hematogenous pyelonephritis. J Infect Dis. 1978 Nov;138(5):664–667. doi: 10.1093/infdis/138.5.664. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Phase variation: genetic analysis of switching mutants. Cell. 1980 Apr;19(4):845–854. doi: 10.1016/0092-8674(80)90075-6. [DOI] [PubMed] [Google Scholar]
- Smith H. The virulence-enhancing factor of mucins. 1. A biological assay of virulence-enhancing activity. Biochem J. 1950 Mar;46(3):352–356. doi: 10.1042/bj0460352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., Edwards K. M., Morris F., McGee Z. A. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J Infect Dis. 1982 Oct;146(4):568–568. doi: 10.1093/infdis/146.4.568. [DOI] [PubMed] [Google Scholar]
- Swaney L. M., Liu Y. P., To C. M., To C. C., Ippen-Ihler K., Brinton C. C., Jr Isolation and characterization of Escherichia coli phase variants and mutants deficient in type 1 pilus production. J Bacteriol. 1977 Apr;130(1):495–505. doi: 10.1128/jb.130.1.495-505.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]