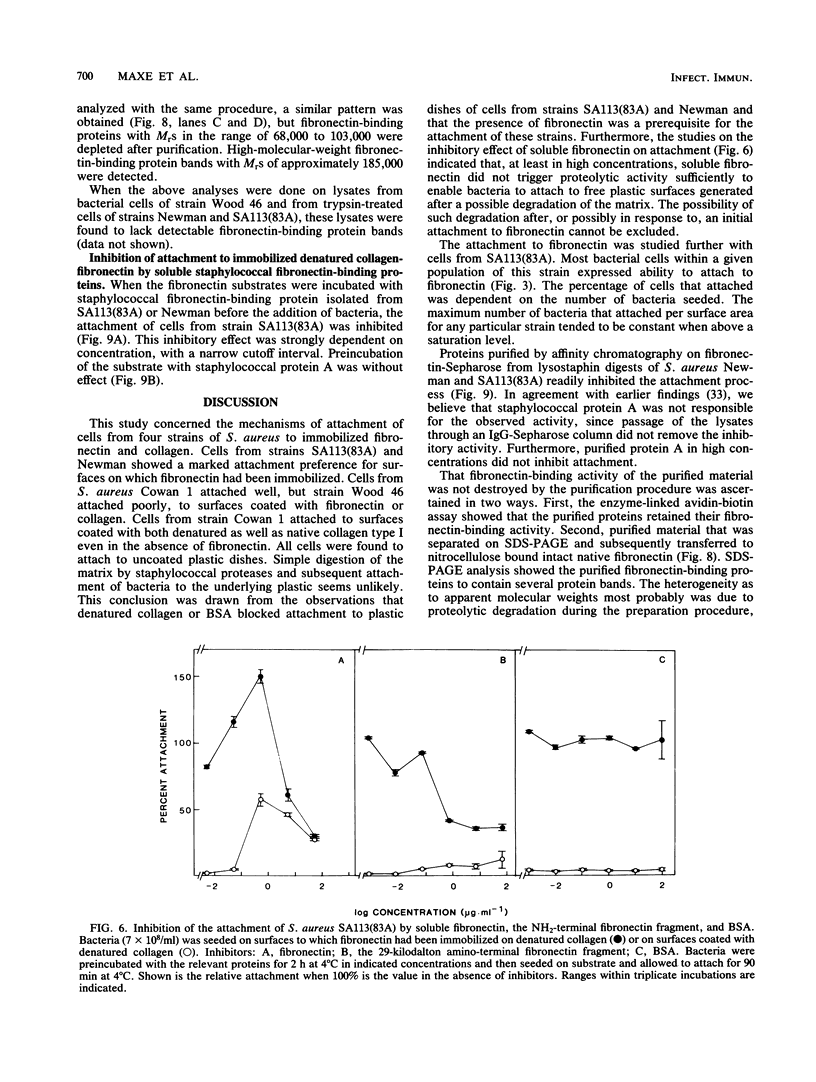
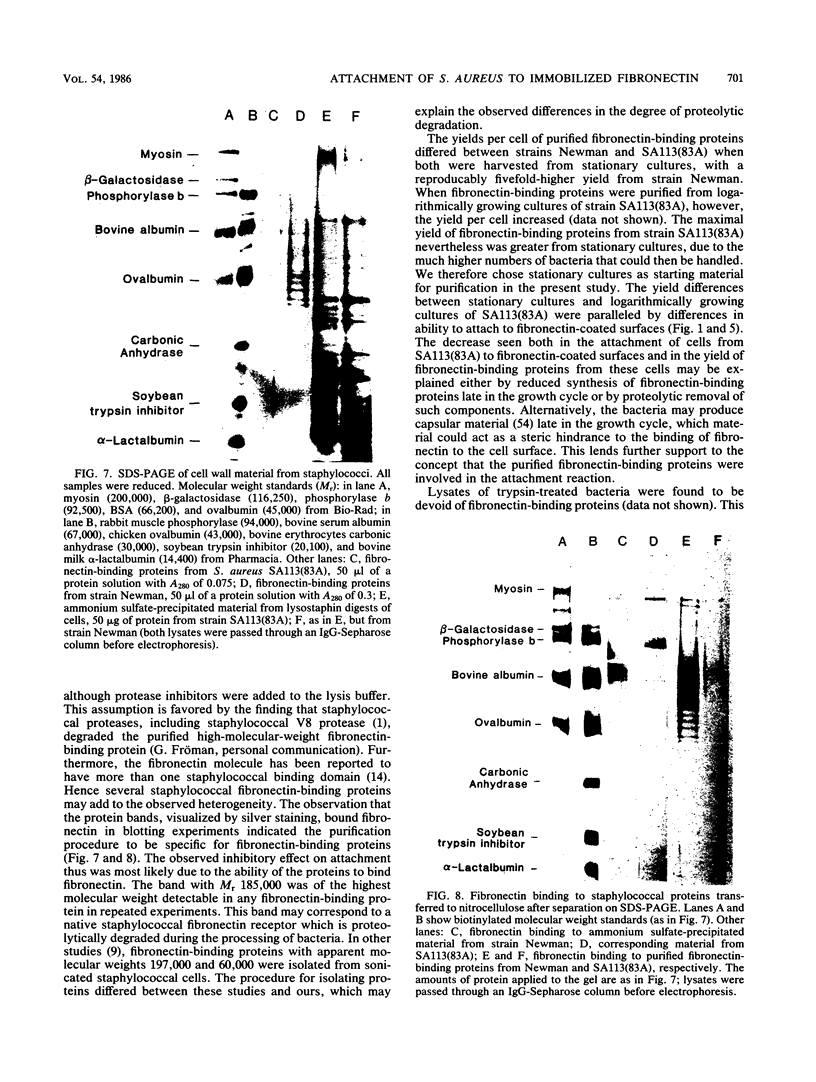
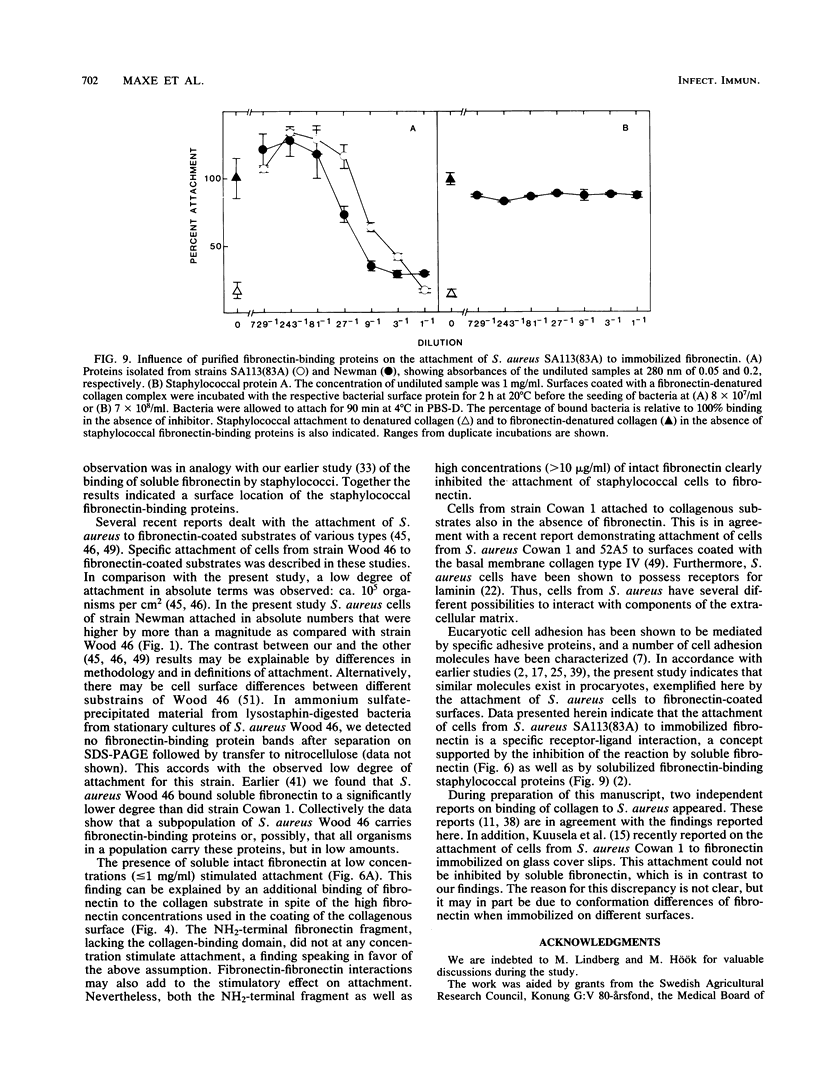
Abstract
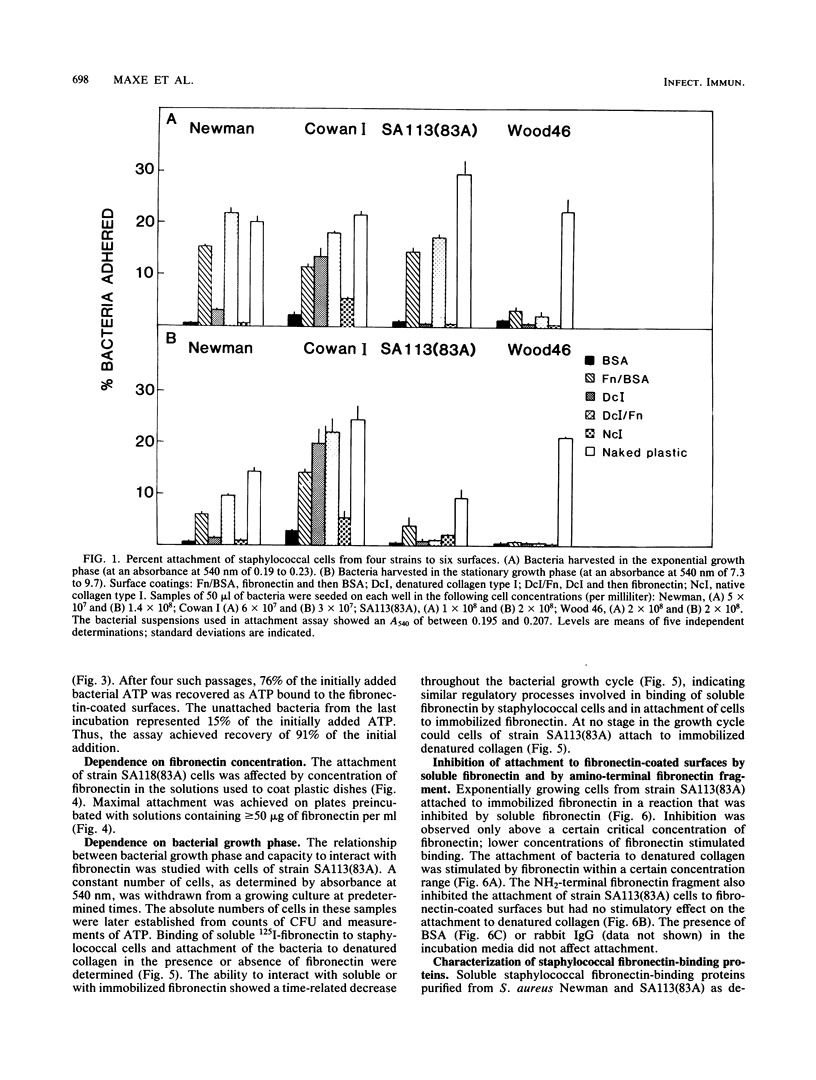
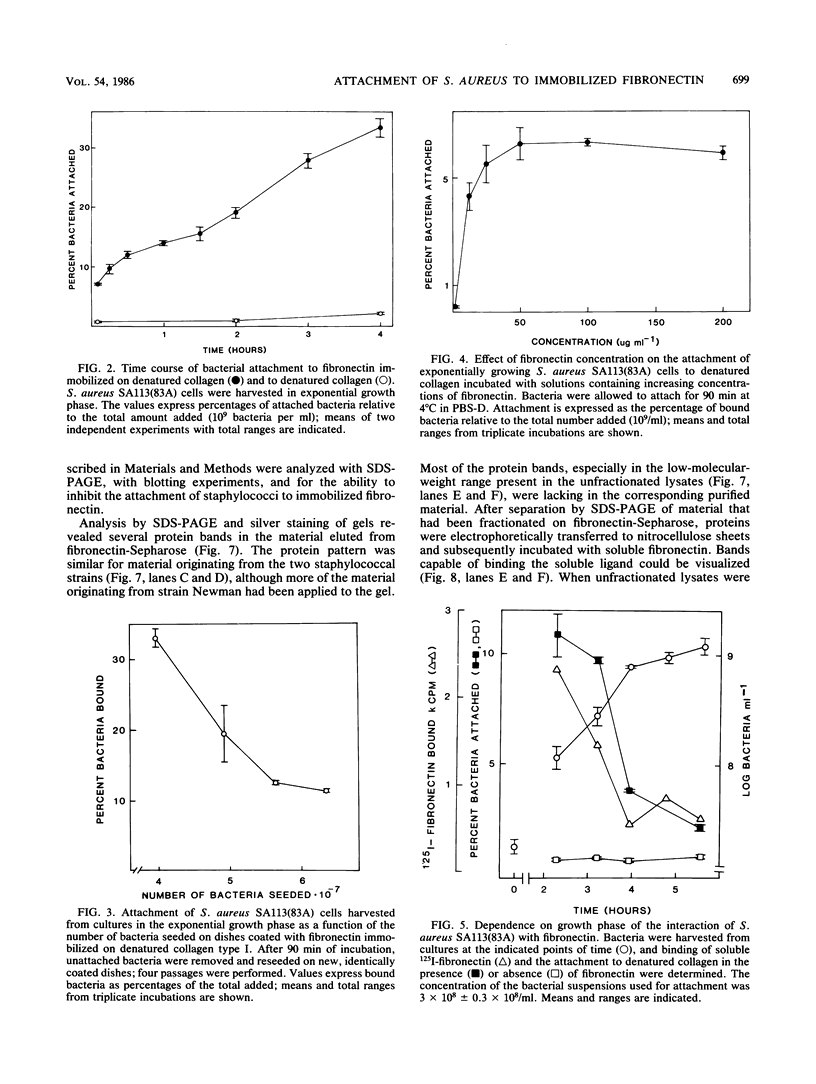
Staphylococcus aureus cells have been shown to possess surface-associated proteins with affinity for soluble fibronectin. We have investigated the ability of these surface proteins to mediate attachment to immobilized fibronectin and collagen. Attachment was quantified by determination of bacterial ATP in a bioluminescence assay. The ability to attach to fibronectin- or collagen-coated plastic surfaces was investigated for four S. aureus strains: Cowan 1, Newman, SA113(83A), and Wood 46. Cells from the different strains varied in their attachment properties, but all cells except those of strain Wood 46 attached readily to substrates coated with fibronectin. Only cells from strain Cowan 1 attached reproducibly to collagen-coated substrates in the absence of fibronectin. The attachment of cells from strain SA113(83A) to fibronectin-coated surfaces was shown to be dependent on time, fibronectin concentration, and bacterial growth phase. Soluble fibronectin or NH2-terminal fibronectin fragment (Mr, 29,000) disturbed the attachment to surfaces coated with fibronectin bound to denatured collagen type I. The attachment process to such substrates was also effectively inhibited by preincubating the substrate with fibronectin-binding proteins isolated from S. aureus Newman and SA113 (83A) and purified with affinity chromatography.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. A. Failure of fibronectin as an opsonin in the host defence system: a case of competitive self inhibition? Lancet. 1983 Nov 5;2(8358):1058–1060. doi: 10.1016/s0140-6736(83)91042-5. [DOI] [PubMed] [Google Scholar]
- Czop J. K., Kadish J. L., Austen K. F. Augmentation of human monocyte opsonin-independent phagocytosis by fragments of human plasma fibronectin. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3649–3653. doi: 10.1073/pnas.78.6.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Freter R., Hagberg L., Hull R., Hull S., Leffler H., Schoolnik G. Inhibition of experimental ascending urinary tract infection by an epithelial cell-surface receptor analogue. Nature. 1982 Aug 5;298(5874):560–562. doi: 10.1038/298560a0. [DOI] [PubMed] [Google Scholar]
- Eriksen H. O., Espersen F., Clemmensen I. Opsonic activity of fibronectin in the phagocytosis of Staphylococcus aureus by polymorphonuclear leukocytes. Eur J Clin Microbiol. 1984 Apr;3(2):108–112. doi: 10.1007/BF02014326. [DOI] [PubMed] [Google Scholar]
- Espersen F., Clemmensen I. Isolation of a fibronectin-binding protein from Staphylococcus aureus. Infect Immun. 1982 Aug;37(2):526–531. doi: 10.1128/iai.37.2.526-531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- Holderbaum D., Spech R. A., Ehrhart L. A. Specific binding of collagen to Staphylococcus aureus. Coll Relat Res. 1985 Jun;5(3):261–271. doi: 10.1016/s0174-173x(85)80016-9. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Holmdahl R., Larsson E., Wigzell H. Role of T lymphocytes in collagen II induced arthritis in rats. Clin Exp Immunol. 1983 Jan;51(1):117–125. [PMC free article] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Attachment of staphylococci and streptococci on fibronectin, fibronectin fragments, and fibrinogen bound to a solid phase. Infect Immun. 1985 Oct;50(1):77–81. doi: 10.1128/iai.50.1.77-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Binding sites for streptococci and staphylococci in fibronectin. Infect Immun. 1984 Aug;45(2):433–436. doi: 10.1128/iai.45.2.433-436.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landwall P. Dialysis culture for the production of extracellular protein A from Staphylococcus aureus A676. J Appl Bacteriol. 1978 Feb;44(1):151–158. doi: 10.1111/j.1365-2672.1978.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Lanser M. E., Saba T. M. Fibronectin as a co-factor necessary for optimal granulocyte phagocytosis of Staphylococcus aureus. J Reticuloendothel Soc. 1981 Nov;30(5):415–424. [PubMed] [Google Scholar]
- Lee D. A., Hoidal J. R., Clawson C. C., Quie P. G., Peterson P. K. Phagocytosis by polymorphonuclear leukocytes of Staphylococcus aureus and Pseudomonas aeruginosa adherent to plastic, agar, or glass. J Immunol Methods. 1983 Sep 30;63(1):103–114. doi: 10.1016/0022-1759(83)90213-2. [DOI] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder E., Vaheri A., Ruoslahti E., Wartiovaara J. Distribution of fibroblast surface antigen in the developing chick embryo. J Exp Med. 1975 Jul 1;142(1):41–49. doi: 10.1084/jem.142.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungh A., Hjertén S., Wadström T. High surface hydrophobicity of autoaggregating Staphylococcus aureus strains isolated from human infections studied with the salt aggregation test. Infect Immun. 1985 Feb;47(2):522–526. doi: 10.1128/iai.47.2.522-526.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Ludwicka A., Jansen B., Wadström T., Pulverer G. Attachment of staphylococci to various synthetic polymers. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Apr;256(4):479–489. doi: 10.1016/s0174-3031(84)80024-4. [DOI] [PubMed] [Google Scholar]
- McNeish A. S., Turner P., Fleming J., Evans N. Mucosal adherence of human enteropathogenic Escherichia coli. Lancet. 1975 Nov 15;2(7942):946–948. doi: 10.1016/s0140-6736(75)90360-8. [DOI] [PubMed] [Google Scholar]
- Miörner H., Myhre E., Björck L., Kronvall G. Effect of specific binding of human albumin, fibrinogen, and immunoglobulin G on surface characteristics of bacterial strains as revealed by partition experiments in polymer phase systems. Infect Immun. 1980 Sep;29(3):879–885. doi: 10.1128/iai.29.3.879-885.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Holmberg O., Kronvall G. Immunoglobulin-binding structure on bovine group G streptococci different from type III Fc receptors on human group G streptococci. Infect Immun. 1979 Jan;23(1):1–7. doi: 10.1128/iai.23.1.1-7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. P., Blobel H. Absorption of human alpha 2-macroglobulin with selected strains of streptococci. Med Microbiol Immunol. 1983;172(1):33–39. doi: 10.1007/BF02123675. [DOI] [PubMed] [Google Scholar]
- Obrink B. Non-aggregated tropocollagen at physiological pH and ionic strength. A chemical and physico-chemical characterization of tropocollagen isolated from the skin of lathyritic rats. Eur J Biochem. 1972 Feb;25(3):563–572. doi: 10.1111/j.1432-1033.1972.tb01729.x. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., Mosher D. F., Olbrantz P. J. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982 Dec 25;257(24):14788–14794. [PubMed] [Google Scholar]
- Proctor R. A., Prendergast E., Mosher D. F. Fibronectin mediates attachment of Staphylococcus aureus to human neutrophils. Blood. 1982 Apr;59(4):681–687. [PubMed] [Google Scholar]
- Rydén C., Rubin K., Speziale P., Hök M., Lindberg M., Wadström T. Fibronectin receptors from Staphylococcus aureus. J Biol Chem. 1983 Mar 10;258(5):3396–3401. [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Hasty D. L., Mason J. M., Beachey E. H. Fibronectin-mediated binding of group A streptococci to human polymorphonuclear leukocytes. Infect Immun. 1982 Aug;37(2):805–810. doi: 10.1128/iai.37.2.805-810.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Switalski L. M., Wadström T. Fibronectin binding to a Streptococcus pyogenes strain. J Bacteriol. 1984 Feb;157(2):420–427. doi: 10.1128/jb.157.2.420-427.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Ljungh A., Rydén C., Rubin K., Hök M., Wadström T. Binding of fibronectin to the surface of group A, C, and G streptococci isolated from human infections. Eur J Clin Microbiol. 1982 Dec;1(6):381–387. doi: 10.1007/BF02019939. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Rydén C., Rubin K., Ljungh A., Hök M., Wadström T. Binding of fibronectin to Staphylococcus strains. Infect Immun. 1983 Nov;42(2):628–633. doi: 10.1128/iai.42.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unny S. K., Middlebrooks B. L. Streptococcal rheumatic carditis. Microbiol Rev. 1983 Mar;47(1):97–120. doi: 10.1128/mr.47.1.97-120.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Water L., Destree A. T., Hynes R. O. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983 Apr 8;220(4593):201–204. doi: 10.1126/science.6338594. [DOI] [PubMed] [Google Scholar]
- Vartio T., Seppä H., Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 1981 Jan 10;256(1):471–477. [PubMed] [Google Scholar]
- Vaudaux P. E., Waldvogel F. A., Morgenthaler J. J., Nydegger U. E. Adsorption of fibronectin onto polymethylmethacrylate and promotion of Staphylococcus aureus adherence. Infect Immun. 1984 Sep;45(3):768–774. doi: 10.1128/iai.45.3.768-774.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P., Suzuki R., Waldvogel F. A., Morgenthaler J. J., Nydegger U. E. Foreign body infection: role of fibronectin as a ligand for the adherence of Staphylococcus aureus. J Infect Dis. 1984 Oct;150(4):546–553. doi: 10.1093/infdis/150.4.546. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Smith D. E., Nguyen B. Y., Hoidal J. R., Wilkinson B. J., Verhoef J., Furcht L. T. Human fibronectin binding to staphylococcal surface protein and its relative inefficiency in promoting phagocytosis by human polymorphonuclear leukocytes, monocytes, and alveolar macrophages. Infect Immun. 1981 Sep;33(3):811–819. doi: 10.1128/iai.33.3.811-819.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Vercellotti G. M., McCarthy J. B., Lindholm P., Peterson P. K., Jacob H. S., Furcht L. T. Extracellular matrix proteins (fibronectin, laminin, and type IV collagen) bind and aggregate bacteria. Am J Pathol. 1985 Jul;120(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Takahashi M., Ohtomo T., Minegishi Y., Ichiman Y., Haga K., Kono E., San Clemente C. L. Application of fluorescent antibody for detecting capsular substances in Staphylococcus aureus. J Appl Bacteriol. 1979 Feb;46(1):147–152. doi: 10.1111/j.1365-2672.1979.tb02592.x. [DOI] [PubMed] [Google Scholar]