Abstract
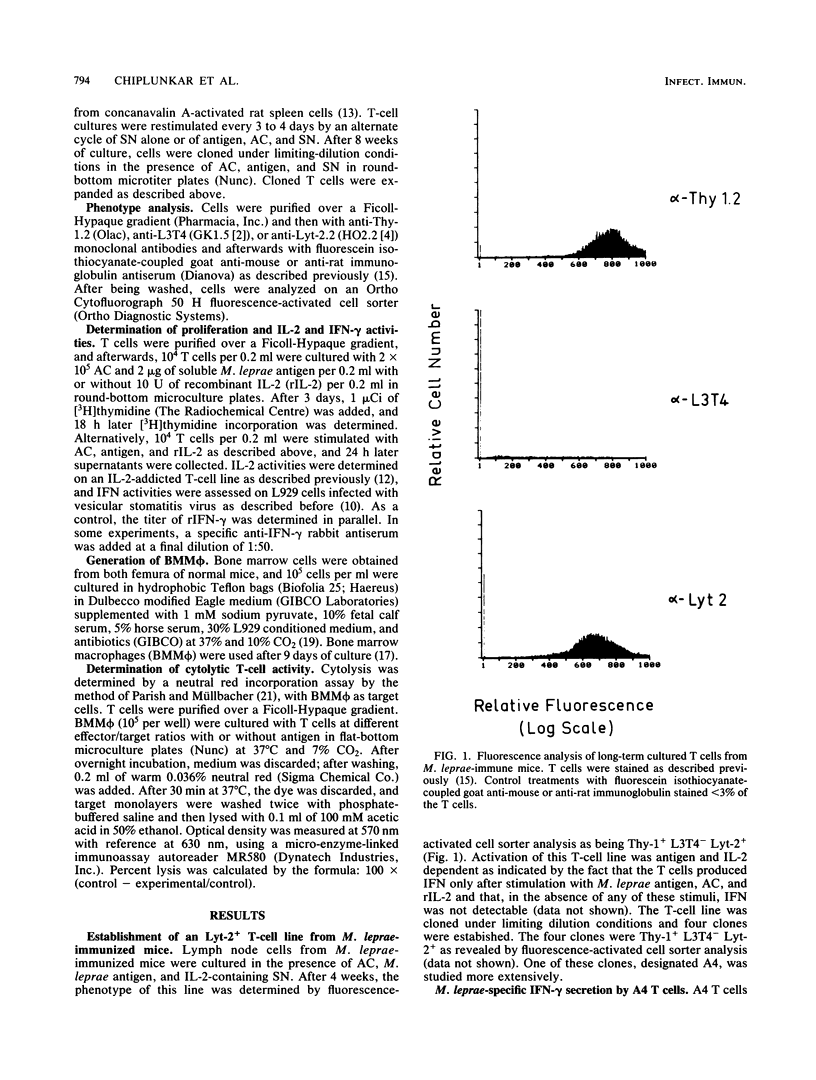
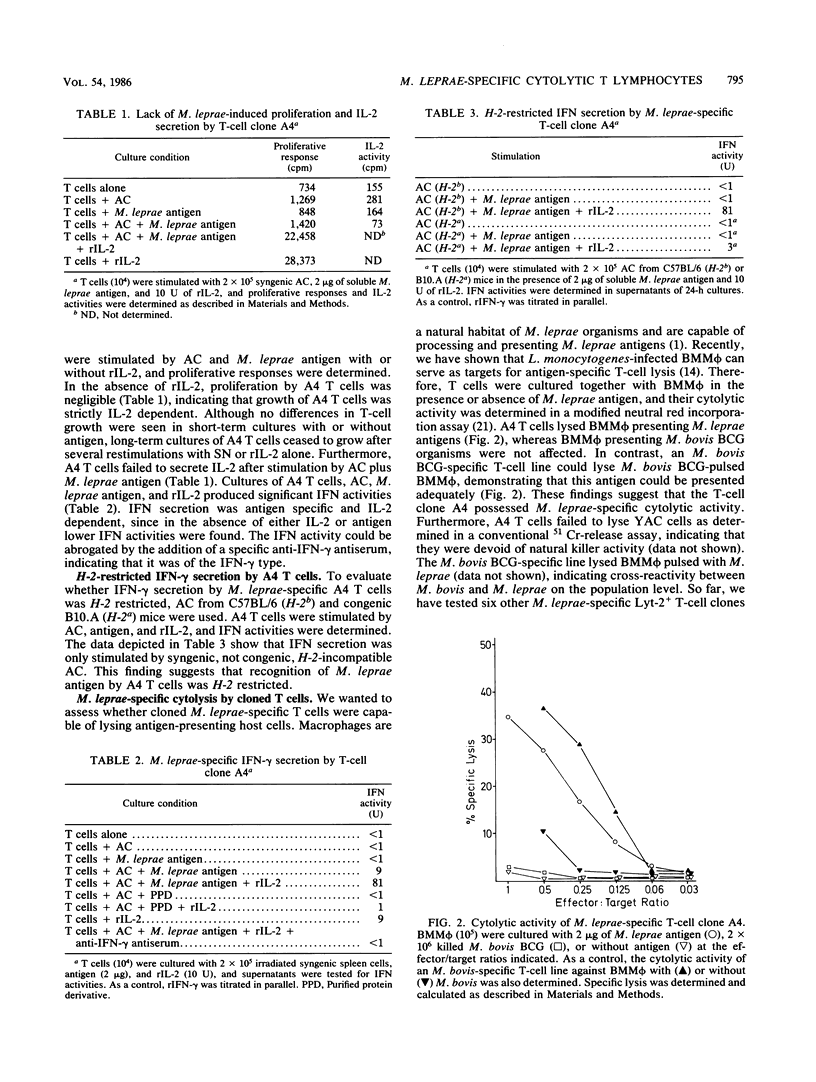
Mice were immunized intradermally with 10(7) irradiated Mycobacterium leprae organisms, and draining lymph nodes were collected after 4 weeks. Lymph node cells were restimulated in vitro with soluble M. leprae antigen and accessory cells. The resulting T-cell line was propagated in vitro in the presence of M. leprae antigen, accessory cells, and interleukin-2-containing supernatants from concanavalin A-stimulated rat spleen cells. Long-term cultured T cells were Thy-1+ L3T4- Lyt-2+ as revealed by analysis with the fluorescence-activated cell sorter. From this line, T-cell clones with the same phenotype were established. The T-cell clone A4 failed to secret interleukin-2 after stimulation with antigen and accessory cells, and its growth depended on exogeneous interleukin-2. A4 T cells produced gamma interferon in an antigen-specific, H-2-restricted, and interleukin-2-dependent way. Importantly, this T-cell clone was capable of lysing bone marrow macrophages presenting M. leprae antigen. Other T-cell clones as well as native Lyt-2+ T cells from M. leprae-immunized mice were also capable of lysing bone marrow macrophages expressing M. leprae antigens. These findings suggest that specific Lyt-2+ T cells participate in the immune response to M. leprae. It is postulated that cytolysis of M. leprae-infected macrophages or Schwann cells contributes to protection against and pathogenesis of leprosy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Emmrich F., Kaufmann S. H. Human T-cell clones with reactivity to Mycobacterium leprae as tools for the characterization of potential vaccines against leprosy. Infect Immun. 1986 Mar;51(3):879–883. doi: 10.1128/iai.51.3.879-883.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Job C. K. Mycobacterium leprae in nerve lesions in lepromatous leprosy. An electron microscopic study. Arch Pathol. 1970 Mar;89(3):195–207. [PubMed] [Google Scholar]
- Kaufmann S. H. Biological activities of a murine T-cell clone with reactivity to Mycobacterium leprae. Cell Immunol. 1984 Jan;83(1):215–220. doi: 10.1016/0008-8749(84)90241-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Brinkmann V. Attempts to characterize the T-cell population and lymphokine involved in the activation of macrophage oxygen metabolism in murine listeriosis. Cell Immunol. 1984 Oct 15;88(2):545–550. doi: 10.1016/0008-8749(84)90186-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Effective antibacterial protection induced by a Listeria monocytogenes-specific T cell clone and its lymphokines. Infect Immun. 1983 Mar;39(3):1265–1270. doi: 10.1128/iai.39.3.1265-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Eichmann K., Müller I., Wrazel L. J. Vaccination against the intracellular bacterium Listeria monocytogenes with a clonotypic antiserum. J Immunol. 1985 Jun;134(6):4123–4127. [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., De Libero G. Listeria monocytogenes-reactive T lymphocyte clones with cytolytic activity against infected target cells. J Exp Med. 1986 Jul 1;164(1):363–368. doi: 10.1084/jem.164.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Immunology of leprosy: new findings, future perspectives. Microb Pathog. 1986 Apr;1(2):107–114. doi: 10.1016/0882-4010(86)90013-6. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Specific Lyt 123 cells are involved in protection against Listeria monocytogenes and in delayed-type hypersensitivity to listerial antigens. J Exp Med. 1979 Oct 1;150(4):1033–1038. doi: 10.1084/jem.150.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerpohl H. G., Lohmann-Matthes M. L., Fischer H. Studies on the activation of mouse bone marrow-derived macrophages by the macrophage cytotoxicity factor (MCF). Eur J Immunol. 1976 Mar;6(3):213–217. doi: 10.1002/eji.1830060313. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Müllbacher A. Automated colorimetric assay for T cell cytotoxicity. J Immunol Methods. 1983 Mar 11;58(1-2):225–237. doi: 10.1016/0022-1759(83)90277-6. [DOI] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., Van Landingham R. M., Ye S. Z. Sensitization or tolerance to Mycobacterium leprae antigen by route of injection. Infect Immun. 1982 Nov;38(2):673–680. doi: 10.1128/iai.38.2.673-680.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Röllinghoff M. T-T-cell interactions during the vitro cytotoxic allograft responses. I. Soluble products from activated Lyl+ T cells trigger autonomously antigen-primed Ly23+ T cells to cell proliferation and cytolytic activity. J Exp Med. 1978 Dec 1;148(6):1523–1538. doi: 10.1084/jem.148.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]


