Abstract
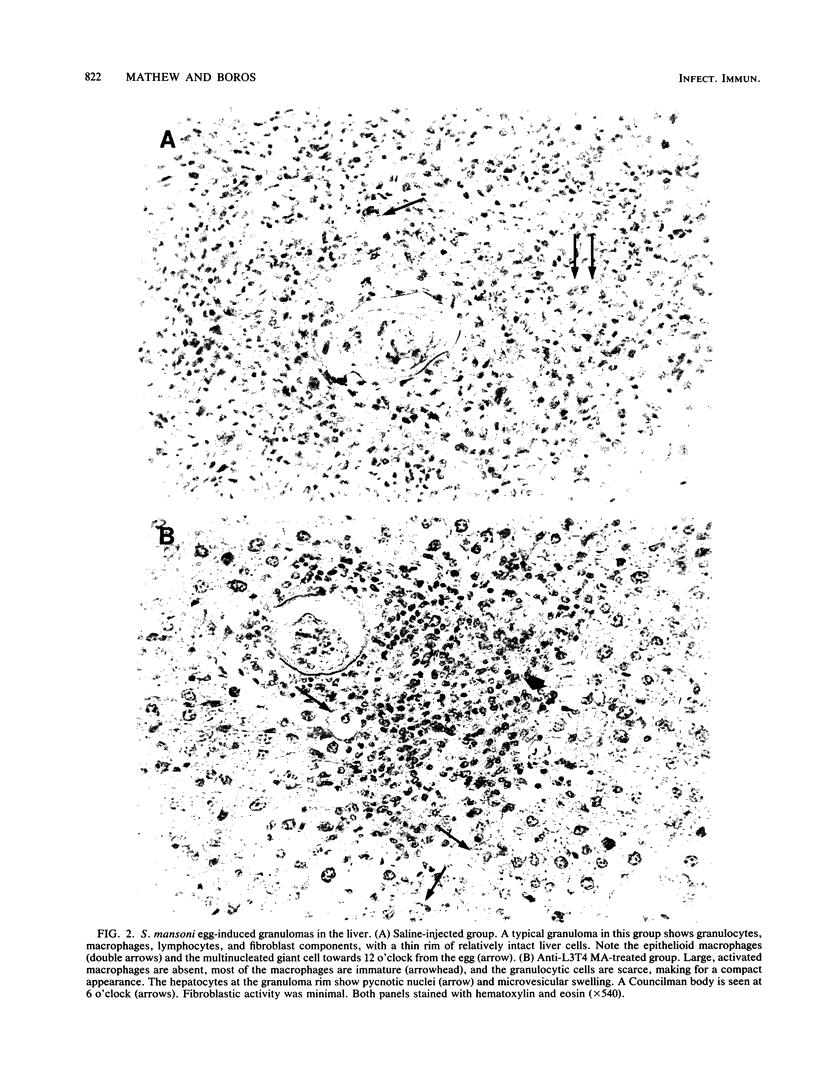
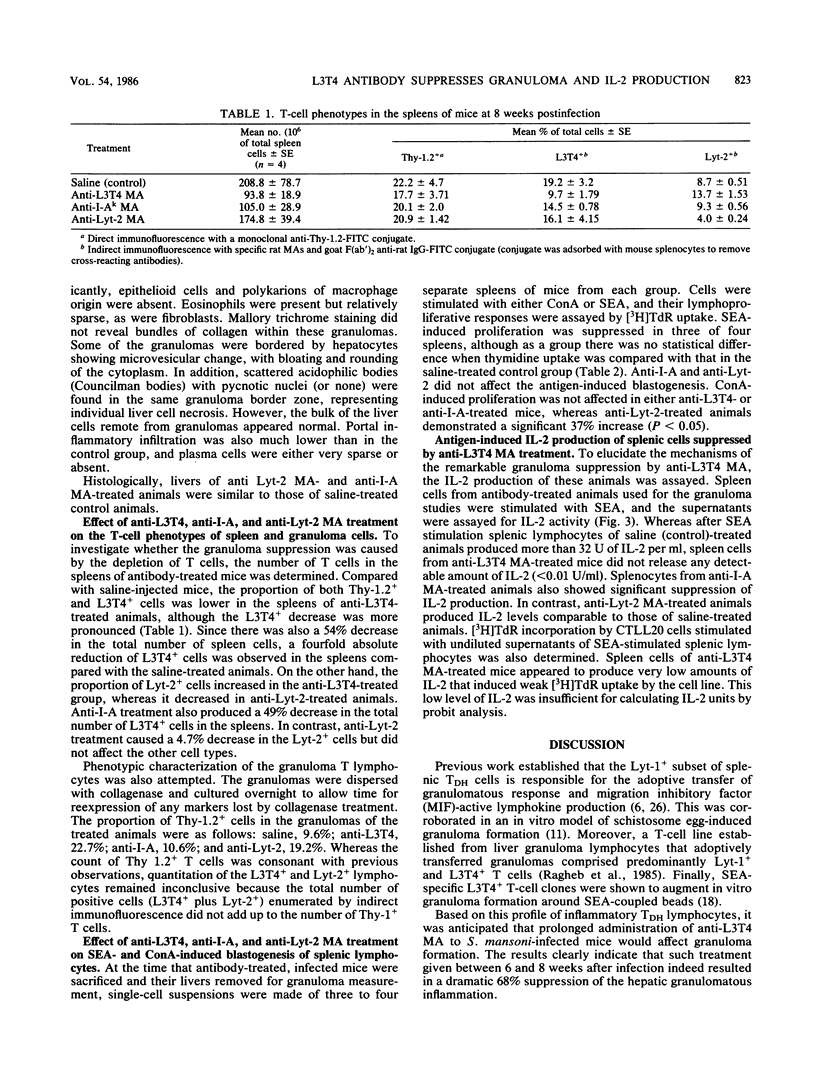
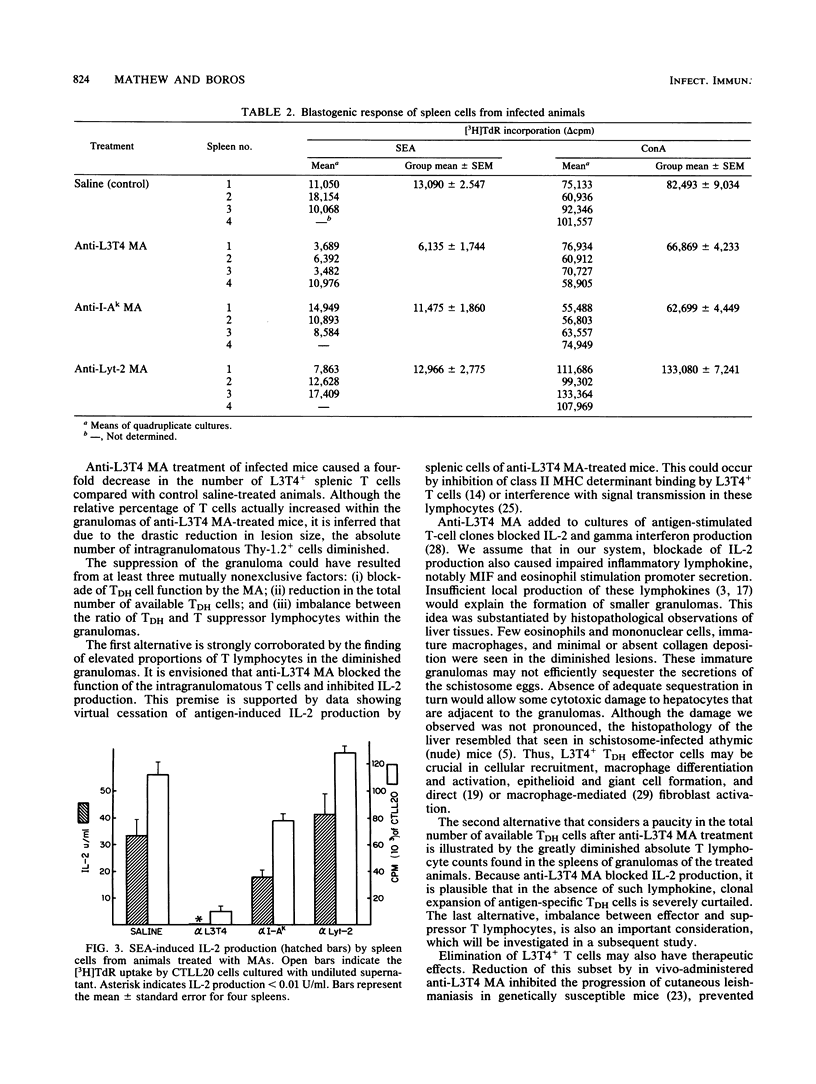
In murine schistosomiasis mansoni, granulomatous inflammation is an immune response that involves egg antigen presentation to T cells in the context of class II major histocompatibility complex determinants and subsequent inflammatory lymphokine production by delayed-hypersensitivity (TDH) lymphocytes. In the present study, monoclonal antibodies directed against L3T4, I-A, and Lyt-2 molecules were injected intraperitoneally into S. mansoni-infected mice to study the role of these membrane antigens in the process of granuloma formation. A dramatic suppression of the hepatic granuloma size and antigen-induced interleukin-2 (IL-2) production by spleen cells was seen in mice that received anti-L3T4 monoclonal antibody treatment. The total number of cells, especially the L3T4+ T cells, was greatly diminished in the spleens. Furthermore, histopathological study of the granulomas in stained liver sections demonstrated the paucity of eosinophils and macrophages, absence of epithelioid cells and multinucleated giant cells, and minimal collagen deposition within the lesions. Damaged hepatocytes were also seen surrounding these ill-formed granulomas. In contrast, anti-I-A monoclonal antibody treatment partially suppressed IL-2 production, although granuloma size and cellular composition remained the same. Mice that received anti-Lyt-2 monoclonal antibody did not show any changes in either IL-2 production or hepatic granulomatous inflammation. The data presented in this paper indicate a crucial role for L3T4 molecules present on a subset of class II major histocompatibility complex-restricted TDH cells in IL-2 production and the generation of the granulomatous response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsden A. F., Boros D. L., Hood A. T. Etiology of the liver granulomatous response in Schistosoma mansoni-infected athymic nude mice. Infect Immun. 1980 Jan;27(1):75–80. doi: 10.1128/iai.27.1.75-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Warren K. S., Pelley R. P. The secretion of migration inhibitory factor by intact schistosome egg granulomas maintained in vitro. Nature. 1973 Nov 23;246(5430):224–226. doi: 10.1038/246224a0. [DOI] [PubMed] [Google Scholar]
- Buchanan R. D., Fine D. P., Colley D. G. Schistosoma mansoni infection in mice depleted of thymus-dependent lymphocytes. II. Pathology and altered pathogenesis. Am J Pathol. 1973 May;71(2):207–218. [PMC free article] [PubMed] [Google Scholar]
- Byram J. E., von Lichtenberg F. Altered schistosome granuloma formation in nude mice. Am J Trop Med Hyg. 1977 Sep;26(5 Pt 1):944–956. doi: 10.4269/ajtmh.1977.26.944. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L., David C. S. Regulation of granulomatous inflammation in murine schistosomiasis. In vitro characterization of T lymphocyte subsets involved in the production and suppression of migration inhibition factor. J Exp Med. 1980 Jun 1;151(6):1398–1412. doi: 10.1084/jem.151.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. 3. Heterologous antilymphocyte serum. Am J Pathol. 1968 Mar;52(3):613–631. [PMC free article] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. II. Thymectomy. Am J Pathol. 1967 Nov;51(5):757–767. [PMC free article] [PubMed] [Google Scholar]
- Doughty B. L., Phillips S. M. Delayed hypersensitivity granuloma formation and modulation around Schistosoma mansoni eggs in vitro. II. Regulatory T cell subsets. J Immunol. 1982 Jan;128(1):37–42. [PubMed] [Google Scholar]
- Elliott D. E., Boros D. L. Schistosome egg antigen(s) presentation and regulatory activity by macrophages isolated from vigorous or immunomodulated liver granulomas of Schistosoma mansoni-infected mice. J Immunol. 1984 Mar;132(3):1506–1510. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Golding H., McCluskey J., Munitz T. I., Germain R. N., Margulies D. H., Singer A. T-cell recognition of a chimaeric class II/class I MHC molecule and the role of L3T4. Nature. 1985 Oct 3;317(6036):425–427. doi: 10.1038/317425a0. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Bedell G. N., Zavala D. C., Monick M., Brady M. Role of interleukin-2 release by lung T-cells in active pulmonary sarcoidosis. Am Rev Respir Dis. 1983 Oct;128(4):634–638. doi: 10.1164/arrd.1983.128.4.634. [DOI] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophils and immune mechanisms: production of the lymphokine eosinophil stimulation promoter (ESP) in vitro by isolated intact granulomas. J Reticuloendothel Soc. 1975 Nov;18(5):283–293. [PubMed] [Google Scholar]
- Lammie P. J., Linette G. P., Phillips S. M. Characterization of Schistosoma mansoni antigen-reactive T cell clones that form granulomas in vitro. J Immunol. 1985 Jun;134(6):4170–4175. [PubMed] [Google Scholar]
- Lammie P. J., Michael A. I., Linette G. P., Phillips S. M. Production of a fibroblast-stimulating factor by Schistosoma mansoni antigen-reactive T cell clones. J Immunol. 1986 Feb 1;136(3):1100–1106. [PubMed] [Google Scholar]
- Phillips S. M., Colley D. G. Immunologic aspects of host responses to schistosomiasis: resistance, immunopathology, and eosinophil involvement. Prog Allergy. 1978;24:49–182. [PubMed] [Google Scholar]
- Phillips S. M., DiConza J. J., Gold J. A., Reid W. A. Schistosomiasis in the congenitally athymic (nude) mouse. I. Thymic dependency of eosinophilia, granuloma formation, and host morbidity. J Immunol. 1977 Feb;118(2):594–599. [PubMed] [Google Scholar]
- Sriram S., Steinman L. Anti I-A antibody suppresses active encephalomyelitis: treatment model for diseases linked to IR genes. J Exp Med. 1983 Oct 1;158(4):1362–1367. doi: 10.1084/jem.158.4.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., Hardy R., Herzenberg L. A., Herzenberg L. A., Lanier L., Lim M., Steinman L. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985 Jan 25;227(4685):415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- Wassmer P., Chan C., Lögdberg L., Shevach E. M. Role of the L3T4-antigen in T cell activation. II. Inhibition of T cell activation by monoclonal anti-L3T4 antibodies in the absence of accessory cells. J Immunol. 1985 Oct;135(4):2237–2242. [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Stadecker M. J., Dinarello C. A., O'Dea J. F. Fibroblast stimulation in schistosomiasis. V. Egg granuloma macrophages spontaneously secrete a fibroblast-stimulating factor. J Immunol. 1984 Jun;132(6):3142–3148. [PubMed] [Google Scholar]