Abstract
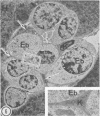
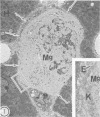
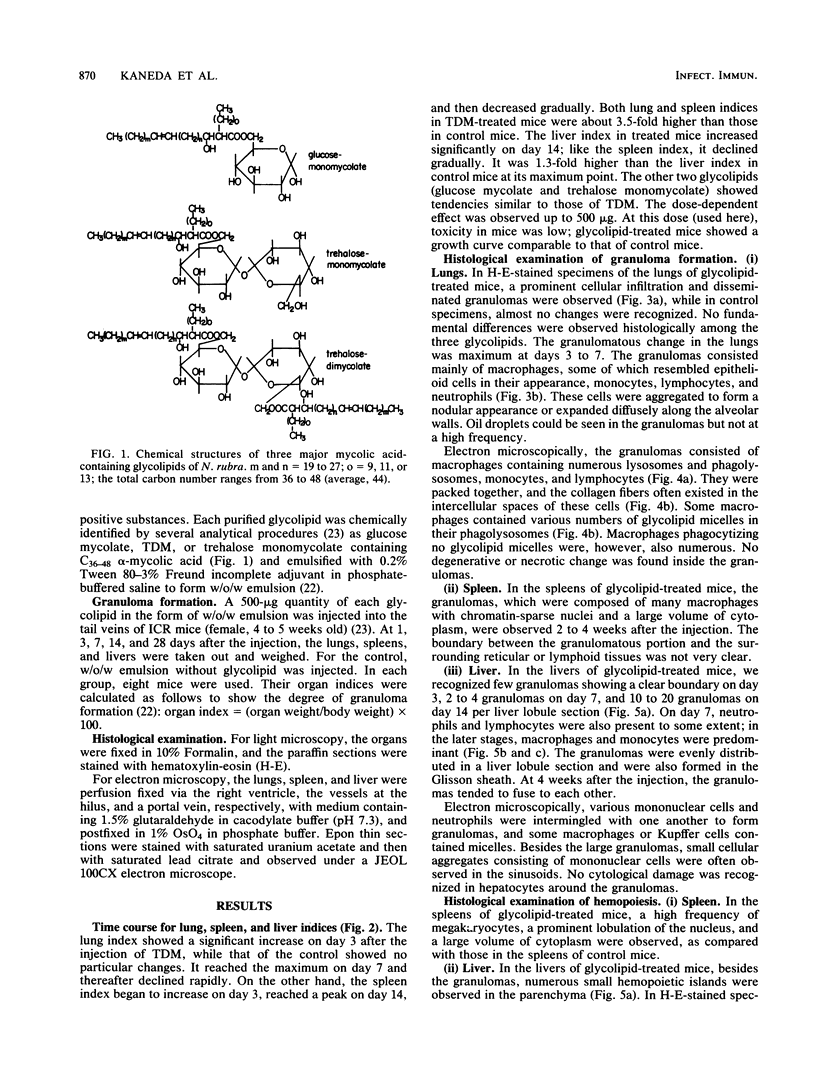
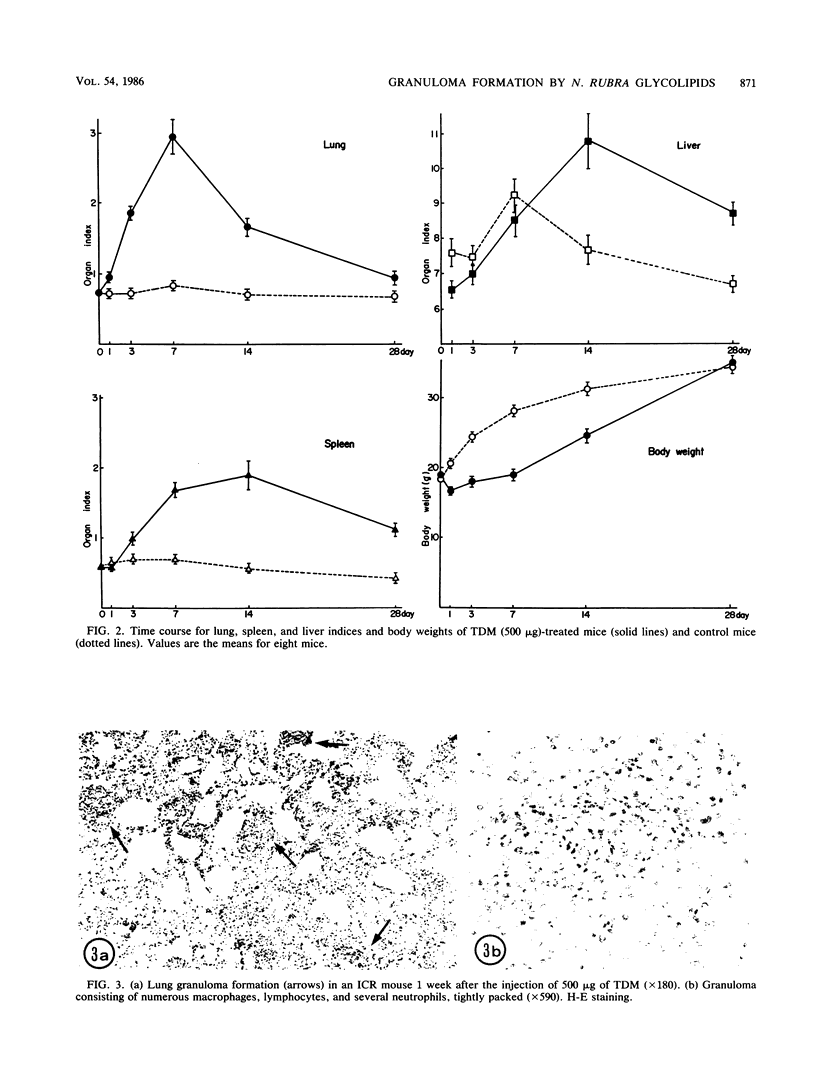
As previously reported (I. Yano, I. Tomiyasu, S. Kitabatake, and K. Kaneda, Acta Leprologica 2:341-349, 1984), Nocardia rubra, one of the nonpathogenic actinomycetes, possesses three classes of mycolic acid-containing glycolipid, i.e., glucose mycolate, trehalose dimycolate, and trehalose monomycolate. The carbon chain length of their mycolic acids is shorter (C36-48) than that in mycobacteria (longer than C70), and the glycolipid consists of only alpha-mycolic acid. One intravenous administration of 500 micrograms of each purified glycolipid to ICR mice in the form of water-in-oil-in-water emulsion without any protein antigens caused prominent granuloma formation in the lungs, spleen, and liver. The lung index in the treated mice was about 3.5 times larger than that in the control mice (given water-in-oil-in-water emulsion only) at 1 week after the injection and then rapidly declined, while spleen and liver indices peaked at 2 weeks after the injection and persisted longer. The granuloma consisted of macrophages, some of which phagocytized glycolipid micelles, lymphocytes, monocytes, and neutrophils. In addition, many small hemopoietic islands were observed in the liver sinusoids, where various immature blood cells were trapped by the prominent cytoplasmic projections of Kupffer cells. The granuloma formation and hemopoiesis observed here are considered to be the most characteristic morphological expression of macrophage activation in these organs. This is the first report to show that such histological changes can be induced by chemically defined and homogeneous mycolic acid-containing glycolipids other than those of mycobacteria.
Full text
PDF
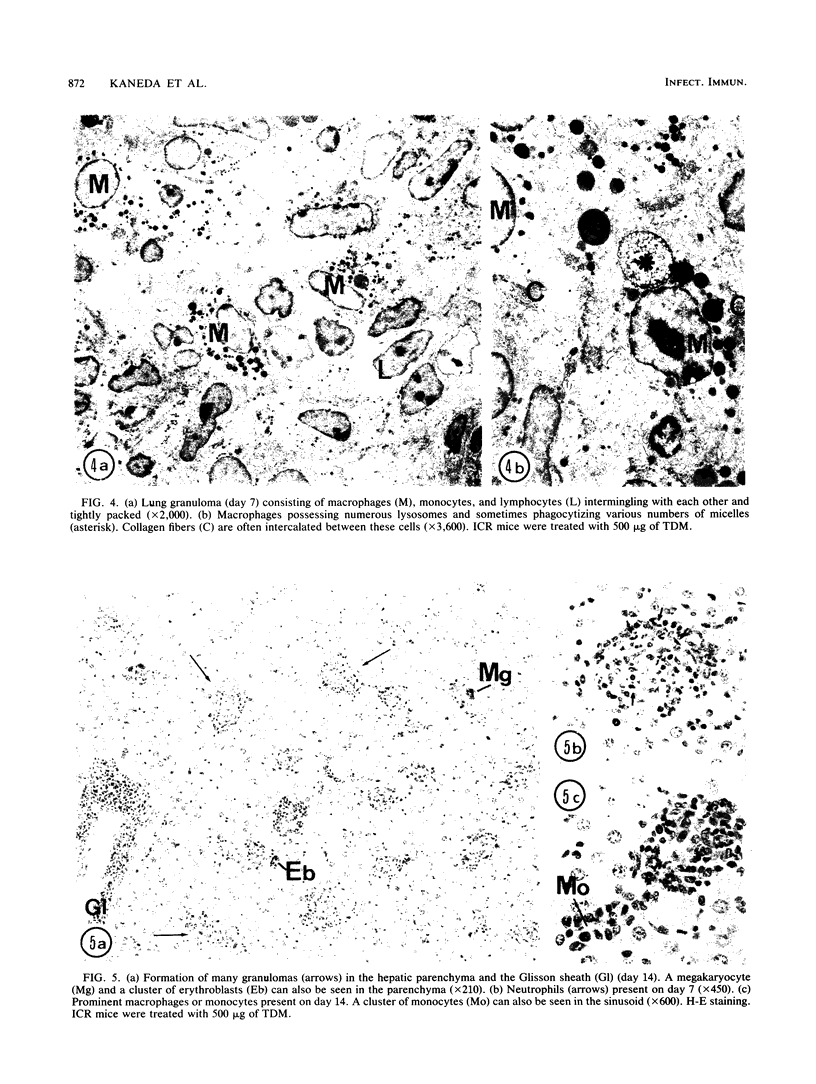
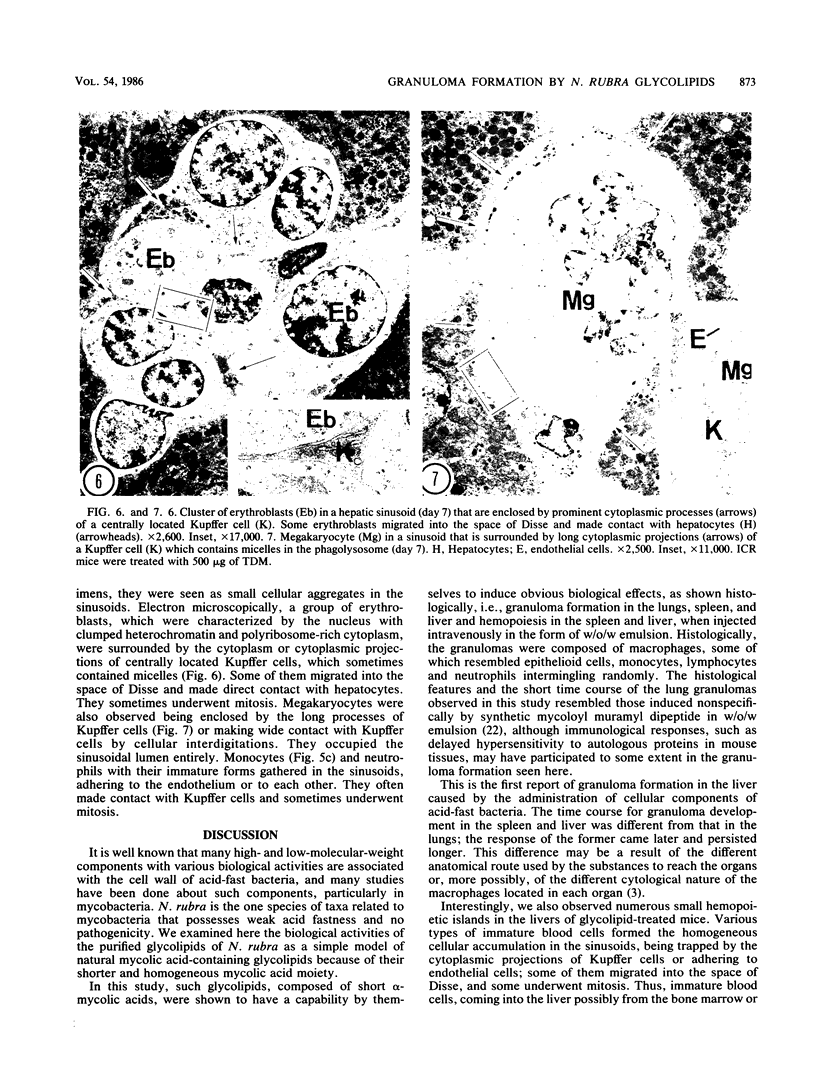


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Adams D. O. The structure of mononuclear phagocytes differentiating in vivo. I. Sequential fine and histologic studies of the effect of Bacillus Calmette-Guerin (BCG). Am J Pathol. 1974 Jul;76(1):17–48. [PMC free article] [PubMed] [Google Scholar]
- Akagawa K. S., Maruyama Y., Takano M., Kasai M., Tokunaga T. A cell surface antigen expressed on mouse lung macrophages. Microbiol Immunol. 1981;25(11):1215–1220. doi: 10.1111/j.1348-0421.1981.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Azuma I., Ribi E. E., Meyer T. J., Zbar B. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst. 1974 Jan;52(1):95–101. doi: 10.1093/jnci/52.1.95. [DOI] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I. S., Yarkoni E., Vilkas E., Adam A., Lederer E. Granuloma formation induced in mice by chemically defined mycobacterial fractions. J Bacteriol. 1969 Oct;100(1):95–102. doi: 10.1128/jb.100.1.95-102.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhles W. C., Jr, Shifrine M. Increased bone marrow production of granulocytes and mononuclear phagocytes induced by mycobacterial adjuvants: improved recovery of leukopoiesis in mice after cyclophosphamide treatment. Infect Immun. 1978 Apr;20(1):58–65. doi: 10.1128/iai.20.1.58-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. Hepatic granulomas induced by glucan. An ultrastructural and peroxidase-cytochemical study. Lab Invest. 1980 Aug;43(2):172–181. [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. Induction of focal hemopoiesis in adult rat liver by glucan, a macrophage activator. A cytochemical and ultrastructural study. Lab Invest. 1980 Feb;42(2):217–224. [PubMed] [Google Scholar]
- Emori K., Tanaka A. Granuloma formation by synthetic bacterial cell wall fragment: muramyl dipeptide. Infect Immun. 1978 Feb;19(2):613–620. doi: 10.1128/iai.19.2.613-620.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama T., Nawa Y., Ezaki T., Kotani M. Effects of estrogen on hepatic hemopoiesis in the adult mouse. Exp Hematol. 1983 Aug;11(7):611–617. [PubMed] [Google Scholar]
- Meyer T. J., Ribi E., Azuma I. Biologically active components from mycobacterial cell walls. V. Granuloma formation in mouse lungs and guinea pig skin. Cell Immunol. 1975 Mar;16(1):11–24. doi: 10.1016/0008-8749(75)90181-1. [DOI] [PubMed] [Google Scholar]
- Ofek I., Bekierkunst A. Chemotactic response of leukocytes to cord factor (trehalose-6,6'-dimycolate). J Natl Cancer Inst. 1976 Dec;57(6):1379–1381. doi: 10.1093/jnci/57.6.1379. [DOI] [PubMed] [Google Scholar]
- Oldham R. K. Biological response modifiers. J Natl Cancer Inst. 1983 May;70(5):789–796. [PubMed] [Google Scholar]
- Reggiardo Z., Shamsuddin A. K. Granulomagenic activity of serologically active glycolipids from Mycobacterium bovis BCG. Infect Immun. 1976 Dec;14(6):1369–1374. doi: 10.1128/iai.14.6.1369-1374.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley M. J., Heather C. J., Brown I., Willoughby D. A. Experimental epithelioid cell granulomas, tubercle formation and immunological competence: an ultrastructural analysis. J Pathol. 1983 Oct;141(2):97–112. doi: 10.1002/path.1711410202. [DOI] [PubMed] [Google Scholar]
- Schlick E., Hartung K., Stevenson H. C., Chirigos M. A. Secretion of colony-stimulating factors by human monocytes and bone marrow cells after in vitro treatment with biological response modifiers. J Leukoc Biol. 1985 May;37(5):615–627. doi: 10.1002/jlb.37.5.615. [DOI] [PubMed] [Google Scholar]
- Strain S. M., Toubiana R., Ribi E., Parker R. Separation of the mixture of trehalose 6,6'-dimycolates comprising the mycobacterial glycolipid fraction, "P3". Biochem Biophys Res Commun. 1977 Jul 25;77(2):449–456. doi: 10.1016/s0006-291x(77)80001-6. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Emori K. Epithelioid granuloma formation by a synthetic bacterial cell wall component, muramyl dipeptide (MDP). Am J Pathol. 1980 Mar;98(3):733–748. [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Williams W. J. Granulomatous inflammation--a review. J Clin Pathol. 1983 Jul;36(7):723–733. doi: 10.1136/jcp.36.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltrout R. H., Mathieson B. J., Talmadge J. E., Reynolds C. W., Zhang S. R., Herberman R. B., Ortaldo J. R. Augmentation of organ-associated natural killer activity by biological response modifiers. Isolation and characterization of large granular lymphocytes from the liver. J Exp Med. 1984 Nov 1;160(5):1431–1449. doi: 10.1084/jem.160.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Kakinuma M., Kato K., Okuyama H., Azuma I. Relationship of anti-tuberculous protection to lung granuloma produced by intravenous injection of synthetic 6-O-mycoloyl-N-acetylmuramyl-L-alanyl-D-isoglutamine with or without specific antigens. Immunology. 1980 Aug;40(4):557–564. [PMC free article] [PubMed] [Google Scholar]
- Yano I., Tomiyasu I., Kitabatake S., Kaneda K. Granuloma forming activity of mycolic acid-containing glycolipids in nocardia and related taxa. Acta Leprol. 1984 Oct-Dec;2(2-4):341–349. [PubMed] [Google Scholar]
- Yarkoni E., Wang L., Bekierkunst A. Stimulation of macrophages by cord factor and by heat-killed and living BCG. Infect Immun. 1977 Apr;16(1):1–8. doi: 10.1128/iai.16.1.1-8.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]