Abstract
Despite recent evidence for sub-stock structuring, North Sea cod are assessed as a single unit. As a consequence, knowledge of sub-stock trends is poor. In particular, there are no recent evaluations of which spawning grounds are active. Here we report results from the first ichthyoplankton survey to cover the whole North Sea. Also, this survey, conducted in 2004, was the first to make extensive use of DNA-based molecular methods to unambiguously identify early developmental stage cod eggs. We compare the findings from the plankton survey with estimated egg production inferred from the distribution of mature cod in contemporaneous trawl surveys. Results from both approaches were in general agreement and showed hot spots of egg production around the southern and eastern edges of the Dogger Bank, in the German Bight, the Moray Firth and to the east of the Shetlands. These areas broadly coincide with known spawning locations from the period 1940 to 1970. We were, however, unable to directly detect significant numbers of cod eggs at the historic spawning ground off Flamborough (northeast coast of England). The results demonstrate that most of the major spawning grounds of cod in the North Sea are still active but that some localized populations may have been reduced to the point where it is now difficult to detect the presence of eggs in the plankton.
Keywords: cod, spawning, ichthyoplankton, North Sea, molecular probes
1. Introduction
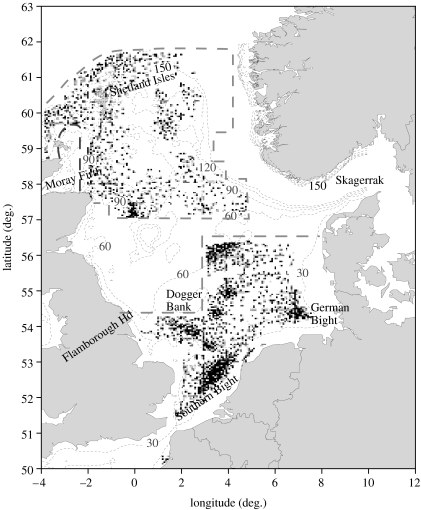
Owing to significant declines in abundance and biomass of many stocks, Atlantic cod are now classified as ‘vulnerable’ by the IUCN (www.iucn.redlist.org). Because of its former importance as a high trophic level predator in marine ecosystems, as well as its value to fisheries, rebuilding these stocks remains a key policy aim in Europe, the USA and Canada (Christensen et al. 2003). Historically, the North Sea contained one of the most productive cod stocks but the present abundance of mature fish is the lowest on record (www.ices.dk). Recent studies suggest that this stock is actually a meta-population comprising a number of resident inshore groups alongside more mobile offshore components (Hutchinson et al. 2001; Wright et al. 2006). Despite this, the stock is assessed as a single management unit covering the North Sea, Skagerrak and eastern English Channel (www.ices.dk). In these assessments, data are aggregated over large areas but this can mask local trends caused by differences in population dynamics (Salvanes et al. 2004), exploitation (Andrews et al. 2006) and environment (Neat & Righton 2006). There is a risk that by focusing at the management unit level, depletion of local sub-stocks may be overlooked with consequent losses of genetic diversity and ecosystem health (Caddy 1999). In recognition of this, regular monitoring of spawning grounds has been recommended as an important component of an ecosystem approach to marine conservation in the North Sea (Scientific Expert Committee 2002). Despite this recommendation, data on which cod spawning areas are currently active in the North Sea are extremely limited. Daan (1978) identified the main spawning areas as around the southern flank of the Dogger Bank and in the Southern Bight (figure 1). However, large areas of the central North Sea remained unsurveyed at that time. This gap was partly addressed by Harding & Nichols (1987) who surveyed some of this area and recorded the presence of cod eggs off Flamborough (England). In later surveys, Heessen & Rijnsdorp (1989) found significant numbers of cod eggs around the southern to eastern flank of the Dogger Bank (January and February 1988). In the northern North Sea, Raitt (1967) reported concentrations of late-stage cod eggs from samples collected east of the Shetlands and in the Moray Firth, and these spawning locations were subsequently confirmed by Heath et al. (1994). Despite the economic and ecological importance of cod in the North Sea, efforts to directly map their spawning grounds using plankton surveys have been fragmentary and there are no data from recent years.
Figure 1.
The most comprehensive available summary map of the spatial distribution of cod eggs according to information collected after 1945 (modified from Daan 1978). Note that the areas outside the dashed lines were not surveyed at the time but that Harding & Nichols (1987) reported additional plankton survey data from the northeast coast of England.
For the purpose of mapping spawning grounds, it is desirable to identify the eggs at the earliest developmental stage. However, early stage cod eggs cannot be visually distinguished from those with similar size and appearance, and in the North Sea these will include eggs of other common species such as haddock Melanogrammus aeglefinus (Heath et al. 1994) and whiting Merlangius merlangus (Fox et al. 2005). This problem has now been overcome using molecular methods (Mork et al. 1983; Taylor et al. 2002; Heffernan et al. 2004; Fox et al. 2005). In 2004, the first ichthyoplankton survey covering the whole North Sea was undertaken using genetic probes to unambiguously identify ‘cod-like’ eggs in their early development stages. In this paper, we report the results and compare the distribution of early stage cod eggs in the plankton with estimated egg production based on the distribution of mature cod in contemporaneous research trawl surveys. It is hoped that these results will form a firm baseline against which future changes in the distribution of cod spawning in the North Sea can be assessed.
2. Material and methods
(a) Ichthyoplankton survey
The timing of the egg surveys was designed to be as close to the historical peak of cod spawning in the different areas as possible (Brander 1994). Since the peak of spawning falls later in the year as one moves north, surveys in the southern North Sea began in late January, the central North Sea was covered during early March and the northern areas surveyed up to the end of March. The basic survey design was for at least two plankton hauls per ICES rectangle (ICES statistical rectangles are 1° of longitude by 0.5° latitude). Plankton were collected using Gulf VII high-speed plankton samplers (Nash et al. 1998) or Bongo nets deployed in double oblique hauls from the sea surface to within 2 m of the seabed (or 100 m in deep stations) at towing speeds of between 3 and 4.5 knots. Salinity and temperature profiles were recorded using conductivity, temperature and depth probes while water volume filtered was calculated from flow metre records. On recovery of the sampler, a subset of up to 100 cod-like eggs (egg with diameter between 1.1 and 1.75 mm and lacking oil globules or other visual features characteristic of other species) was sorted from the catch and these eggs individually preserved in ethanol (Taylor et al. 2002; Fox et al. 2005). These eggs were subsequently analysed using TaqMan probes specific for cod, haddock and whiting (Taylor et al. 2002). Prior to molecular analysis, 614 hatchery-spawned cod and haddock eggs were included in the sample series as blind control standards. At sea, the remainder of each plankton sample was fixed in 4% acetate-buffered formaldehyde and fish eggs subsequently sorted and identified using traditional visual methods (Russell 1976).
(b) Modelling of the ichthyoplankton data
The ratio of identified species from TaqMan results (cod, haddock, whiting or other species with cod-like eggs) was modelled as a multinomial distribution. The numbers of eggs of each species identified in the gene-probes subsample were related to geographical position using natural splines with 24 d.f. for latitude, longitude and the interaction of latitude and longitude (Hastie & Tibshirani 1990). Spline smoothness was based upon shelving in the rate of improvement of model fit with increasing degrees of freedom and by consideration of residual plots. The fitted model was used to predict the proportions of the target species at all stations. The predicted species ratio at each station location was then used to apportion the total number of stage I cod-like eggs found at that station between cod, haddock, whiting or other species. Stage I cod egg abundance data were converted to abundance per square metre of sea surface by dividing by the water volume filtered and multiplying by the depth sampled. Daily egg production per square metre of sea surface was then calculated by dividing egg abundance at each station by the stage I temperature-dependent duration derived from laboratory experiments (Thompson & Riley 1981; Geffen et al. 2006). This procedure standardized for temperature differences between the different surveys. Modelling was carried out in R v. 2.2.0 (R Development Core Team 2005). In order to show the original ichthyoplankton data and to facilitate comparison with results from the trawl surveys, daily stage I cod egg production was plotted both by plankton station and as the average per ICES rectangle using Surfer v. 8 (Golden Software, Inc., Golden, CO, USA).
(c) Egg production from trawl survey data in 2004
The distribution of cod egg production in 2004 was also estimated indirectly based on catch data from the quarter 1, ICES International Bottom Trawl Survey (IBTS). The Q1 IBTS survey is undertaken between January and March and comprises one or two half-hour tows per ICES rectangle using standardized gear (Grande Ouverture Verticale trawl). Potential egg production of cod caught in each ICES rectangle was estimated as
| (2.1) |
where Njl is the total number of cod caught in ICES statistical rectangle j and cm length class l and f is the proportion of females (based on the total survey). A maturity-length ogive for the 2004 survey data was fitted by logistic regression to give m, the proportion mature. PF, the potential fecundity at length was taken from Yoneda & Wright (2004).
Potential egg production per ICES rectangle (Er) was then estimated as
| (2.2) |
where Ej is taken from equation (2.1); Ar is the total sea surface area (km−2) of seabed less than 250 m depth within each ICES rectangle (estimated from Digbath 250 implemented within ARC GIS); and Aj is the area swept (km−2) based on a predetermined ‘effective gear width’ (wing spread for the GOV trawl was estimated to be 22 m; Reid et al. 2000) and distance of the survey tows.
3. Results
(a) Ichthyoplankton survey
In total, 502 plankton hauls were made and 8865 cod-like eggs identified using TaqMan probes. Analysis of the 614 embedded hatchery-spawned cod and haddock eggs indicated a low level of false positives and confirmed that the TaqMan method correctly identified more than 95% of the blind standards. The size range of eggs from the survey identified by TaqMan as cod was 1.28–1.63 mm (95 percentiles of size distribution) and for haddock was 1.19–1.62 mm (figure 2), consistent with expected egg sizes (Russell 1976). The maximum size of TaqMan identified whiting eggs was 1.83 mm, which was larger than suggested by Russell (1976) who quoted a min–max size range of 0.97–1.32 mm. Because TaqMan probes have so far only been developed for cod, haddock and whiting, the assay can produce negative results due to the presence of eggs of other species. Eggs falling into this category tended to lie at the lower end of the size range (figure 2). The DNA from a few of these eggs was sequenced and identified as coming from saithe (Pollachius virens), a species common in the northern North Sea. Over the whole survey, 17.6% of TaqMan identified eggs were cod, 48.3% were haddock, 22.2% whiting and 12.0% other species.
Figure 2.
Size frequency distributions of the cod-like eggs subsampled from the ichthyoplankton survey in 2004 and positively identified using TaqMan probes compared with the size frequency distribution of all cod-like eggs in the bulk plankton samples.
(b) Distribution of stage I cod eggs from the ichthyoplankton survey
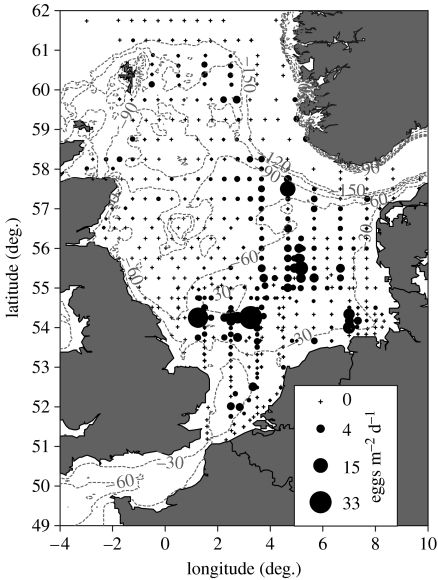
The maximum daily production of stage I cod eggs recorded was 33 m−2 d−1 at a station to the southeast of the Dogger Bank (figure 3). The main concentrations of stage I cod eggs were also found around the southern and eastern edges of the Dogger Bank with another smaller patch in the German Bight. Rather low abundances of cod eggs were found in the Southern Bight and north of latitude 58° but significant numbers of eggs were found off the Moray Firth and to the east of the Shetland Islands.
Figure 3.
The distribution of stage I cod eggs from the 2004 ichthyoplankton survey. The area of the solid circles is proportional to the daily production of cod eggs at each station. Crosses indicate where a plankton sample was collected but no stage I cod eggs were found. Dashed contour lines show the bathymetry in metres.
(c) Egg production estimated from trawl surveys
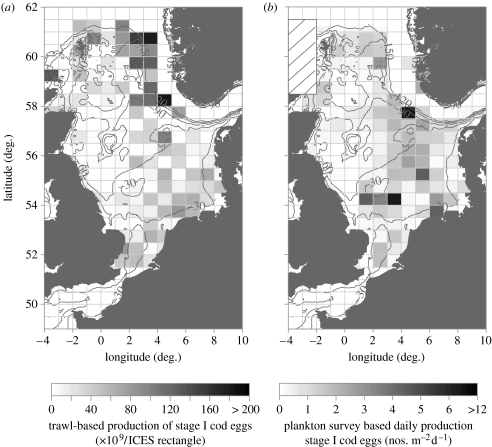
The expected pattern of cod egg production (figure 4a) based on the distribution of mature cod in the trawl survey was broadly similar to that directly observed by the ichthyoplankton survey (figure 4b). In particular, cod eggs were found in the Southern Bight, German Bight, Moray Firth and to the east of the Shetlands as predicted. However, the presence of some mature cod off Flamborough suggested that there should have been spawning in that area, but this was not detected in the ichthyoplankton survey. In addition, high densities of mature-sized cod in the northeastern North Sea close to the Norwegian Trench (north of latitude 59.5° N and east of longitude 002° E) suggested that this region should also have had high levels of egg production. This was not reflected in the direct observations, although some cod eggs were found in this area (figures 3 and 4b).
Figure 4.
(a) The distribution of cod egg production per ICES rectangle in 2004 based on Q1 trawl survey data. (b) The distribution of stage I cod egg production averaged to ICES rectangle resolution from the 2004 ichthyoplankton survey. Cross-hatched area indicates where no plankton samples were collected.
4. Discussion
At the water temperatures recorded, cod eggs will take approximately 3 days to reach the end of stage I (Thompson & Riley 1981). Dispersion during this time should be about the same as the inter-station spacing so the plankton map (figure 3) should provide a reliable indication of spawning locations. The distribution of stage I cod eggs from the 2004 ichthyoplankton survey closely resembled the partial composite of North Sea cod spawning grounds (figure 1) produced by Daan (1978). That composite contained a large unsurveyed region, including the area off Flamborough Head. Later, plankton surveys (Harding & Nichols 1987) recorded cod eggs in this area thus confirming local reports of the presence of mature cod. Harding & Nichols also reported concentrations of stage I cod eggs off the western edge of the Dogger Bank. However, this conclusion was based on egg appearance and size only and some of these cod eggs may have been confused with those of whiting.
Comparing the 2004 ichthyoplankton survey results with predicted egg production based on Q1 trawl survey data also shows general agreement. For the central and southern North Sea, the trawl survey data show concentrations of mature cod in the southern Bight, German Bight and in an arc from the south to the northeast of the Dogger Bank. Active spawning was confirmed by the plankton surveys in these areas, although the highest concentrations of eggs were closer to the edge of the Dogger Bank than suggested by the trawl data. In the northern North Sea, the locations of cod eggs in the plankton samples were also broadly consistent with the patterns reported by Raitt (1967) and Heath et al. (1994). However, the high levels of production predicted by the trawl survey data at the edge of the Norwegian Trench (north of latitude 59.5° N and east of longitude 2° E) were not confirmed by direct observations. Our main conclusion is that most of the historical spawning grounds identified by Daan (1978) were still active in 2004. However, we failed to find significant numbers of cod eggs off Flamborough Head and levels of egg production in the Southern Bight also seemed low.
Although the ichthyoplankton survey was successful in covering the entire North Sea, it was not possible to directly estimate total annual egg production since this would have required repeated surveys throughout the spawning season (Armstrong et al. 2001). The timing of surveys in each area of the North Sea was designed to coincide with the presumed peak in cod egg production (Brander 1994), but it is possible that the sampling missed the peak in some regions. However, since the spawning duration for cod in sub-areas of the North Sea is two to three months (Blanchard et al. 2005), one would expect to find eggs even if the peak had been missed. Our failure to find large concentrations of cod eggs at the edge of the Norwegian Trench where mature cod were caught by trawl therefore requires further investigation.
The application of modern molecular methods holds the promise of rapid, unambiguous identification of cryptic species or life stages (Costa et al. 2007). However, applications to large-scale distribution mapping of marine plankton have been hampered by the need to analyse very large numbers of specimens, by the relatively high per-sample cost of molecular identification and by the time and specialist skills needed (Lindeque et al. 2006). The present results are, to our knowledge, the first to apply modern molecular methods to map the distribution of the cryptic stage of a marine fish at the scale of a large marine ecosystem. This was made possible by using the semi-automated TaqMan method that allowed relatively rapid sample processing (Taylor et al. 2002). Nevertheless, we were still forced to subsample the cod-like eggs from the plankton hauls to reduce the amount of molecular analysis to manageable and affordable proportions. Despite advances in DNA bar coding, robotics, high-speed sequencing and DNA chips, molecular methods should be viewed as a valuable addition to traditional taxonomic skills, rather than a replacement (Hebert et al. 2003). Combining visual microscopy with molecular analysis of subsamples is likely to remain the only practicable approach for large-scale applications in marine plankton studies for the foreseeable future (Lindeque et al. 2006).
In conclusion, the application of modern molecular techniques combined with traditional plankton survey methods allowed us to map the occurrence of early stage cod eggs across the whole North Sea for the first time. This provided direct confirmation of spawning activity in most areas where mature cod were found. This confirms that the major historical spawning grounds for North Sea cod were still active in 2004 but some localized populations may now have been reduced to levels where it is difficult to find cod eggs in the plankton. These populations could be at particular risk of severe depletion and may require targeted conservation measures.
Acknowledgments
The survey was coordinated by the Planning Group on North Sea Egg Surveys (PGEGGS) of ICES. We are indebted to the officers and crew of all the research vessels involved for their efforts, often under arduous winter conditions and the work of all staff at the various institutes who undertook the sorting and identification of the plankton samples. The contributions of the individual institutes were funded through their national research programmes. The molecular analyses were supported by Defra (UK).
References
- Andrews J.M, Gurney W.S.C, Heath M.R, Gallego A, O'Brien C.M, Barby C, Tyldesley G. Modelling the spatial demography of Atlantic cod (Gadus morhua) on the European continental shelf. Can. J. Fish. Aquat. Sci. 2006;63:1027–1048. doi:10.1139/F06-006 [Google Scholar]
- Armstrong M.J, et al. An application of the annual egg production method to estimate spawning biomass of cod (Gadus morhua L.), plaice (Pleuronectes platessa L.) and sole (Solea solea L.) in the Irish Sea. ICES J. Mar. Sci. 2001;58:183–203. doi:10.1006/jmsc.2000.1001 [Google Scholar]
- Blanchard, J. L., Heffernan, O. A. & Fox, C. J. 2005 North Sea cod. ICES cooperative research report, 274, pp. 76–88.
- Brander K.M. The location and timing of cod spawning around the British Isles. J. Mar. Sci. 1994;51:71–89. doi:10.1006/jmsc.1994.1007 [Google Scholar]
- Caddy J.F. Fisheries management in the twenty-first century: will new paradigms apply? Rev. Fish Biol. Fish. 1999;9:1–43. doi:10.1023/A:1008829909601 [Google Scholar]
- Christensen V, Guénette S, Heymans J.J, Walters C.J, Watson R, Zeller D, Pauly D. Hundred-year decline of North Atlantic predatory fishes. Fish Fish. 2003;4:1–24. doi:10.1046/j.1467-2979.2003.00103.x [Google Scholar]
- Costa F.O, deWaard J.R, Boutillier J, Ratnasingham S, Dooh R.T, Hajibabael M, Hebert P.D.N. Biological identificaions through DNA barcodes: the case of the Crustacea. Can. J. Fish. Aquat. Sci. 2007;64:272–295. doi:10.1139/F07-008 [Google Scholar]
- Daan N. Changes in cod stocks and cod fisheries in the North Sea. Rapp. P-V Réun. Cons. Int. Explor. Mer. 1978;172:39–57. [Google Scholar]
- Fox C.J, Taylor M.I, Pereyra R, Villasana-Ortiz M.I, Rico C. TaqMan DNA technology confirms likely over-estimation of cod (Gadus morhua L.) egg abundance in the Irish Sea: implications for the assessment of the cod stock and mapping of spawning areas using egg based methods. Mol. Ecol. 2005;14:879–884. doi: 10.1111/j.1365-294X.2005.02439.x. doi:10.1111/j.1365-294X.2005.02439.x [DOI] [PubMed] [Google Scholar]
- Geffen A.J, Fox C.J, Nash R.D.M. Temperature-dependent development rates of cod Gadus morhua eggs. J. Fish Biol. 2006;69:1–21. doi:10.1111/j.1095-8649.2006.01181.x [Google Scholar]
- Harding, D. & Nichols, J. H. 1987 Plankton surveys off the north-east coast of England in 1976: an introductory report and summary of results. Fisheries research technical report, no. 86, pp. 55.
- Hastie T.J, Tibshirani R.J. Chapman and Hall; London, UK: 1990. Generalized additive models. [DOI] [PubMed] [Google Scholar]
- Heath M, Rankine P, Cargill L.H. Distribution of cod and haddock eggs in the North Sea in 1992 in relation to oceanographic features and compared with distributions in 1952–1957. ICES Mar. Sci. Symp. 1994;198:438–439. [Google Scholar]
- Hebert P.D.N, Cywinska A, Ball S.L, deWaard J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. doi:10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heessen H.J.L, Rijnsdorp A.D. Investigations on egg production and mortality of cod (Gadus morhua L.) and plaice (Pleuronectes platessa L.) in the southern and eastern North Sea in 1987 and 1988. Rapp. P-V Réun. Cons. Int. Explor. Mer. 1989;191:15–20. [Google Scholar]
- Heffernan O.A, Danilowicz B.S, Milligan S.P. Determination of species-specific spawning distributions of commercial finfish in the Irish Sea using a biochemical protein-based method. Mar. Ecol. Prog. Ser. 2004;284:279–291. doi:10.3354/meps284279 [Google Scholar]
- Hutchinson W.F, Carvalho G.R, Rogers S.I. Marked genetic structuring in localised spawning populations of cod Gadus morhua in the North Sea and adjoining waters, as revealed by microsatellites. Mar. Ecol. Prog. Ser. 2001;233:251–260. doi:10.3354/meps223251 [Google Scholar]
- Lindeque P.K, Hay S.J, Heath M.R, Ingvarsdottir A, Rasmussen J, Smerdon G.R, Wanier J.J. Integrating conventional microscopy and molecular analysis to analyse the abundance and distribution of four Calanus congeners in the North Atlantic. J. Plankton Res. 2006;28:221–238. doi:10.1093/plankt/fbi115 [Google Scholar]
- Mork J, Solemdal P, Sundes G. Identification of marine fish eggs: a biochemical genetics approach. Can. J. Fish. Aquat. Sci. 1983;40:361–369. [Google Scholar]
- Nash R.D.M, Dickey-Collas M, Milligan S.P. Descriptions of the Gulf VII/PRO-NET and MAFF/Guildline unencased high-speed plankton samplers. J. Plankton Res. 1998;20:1915–1926. doi:10.1093/plankt/20.10.1915 [Google Scholar]
- Neat F, Righton D. Warm water occupancy by North Sea cod. Proc. R. Soc. B. 2006;274:789–798. doi: 10.1098/rspb.2006.0212. doi:10.1098/rspb.2006.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team 2005 R, system for statistical computing, the R Foundation. See http://www.r-project.org
- Raitt, D. F. S. 1967 Cod spawning in Scottish waters. Preliminary investigations. C.M. 1967/F:29, 3+13 figures pp.
- Reid, D. G., Beare, D. J., Mahe, J.-C., Connolly, P., Davis, C. G. & Newton, A. W. 2000 Quantifying variability in gear performance on IBTS surveys: swept area and volume with depth. ICES CM 2000/K:28, 9 pp.
- Russell F.S. Academic Press; London, UK: 1976. The eggs and planktonic stages of British marine fishes. [Google Scholar]
- Salvanes A.G, Skæraasen J.E, Nilsen T. Sub-populations of coastal cod with different behaviour and life-history strategies. Mar. Ecol. Prog. Ser. 2004;267:241–251. doi:10.3354/meps267241 [Google Scholar]
- Scientific Expert Committee 2002 Priority issues for North Sea ecosystem management. In Scientific Expert Conference related to the 5th North Sea Conference, Institute for Marine Research, Bergen, Norway, 20–22 February 2002, 2 pp
- Taylor M.I, Fox C.J, Rico I, Rico C. Species-specific TaqMan probes for simultaneous identification of cod (Gadus morhua L.), haddock (Melanogrammus aeglefinus L.) and whiting (Merlangius merlangus L.) Mol. Ecol. Notes. 2002;2:599–601. doi:10.1046/j.1471-8286.2002.00269.x [Google Scholar]
- Thompson B.M, Riley J.D. Egg and larval development studies in the North Sea cod (Gadus morhua L.) Rapp. P-V Réun. Cons. Int. Explor. Mer. 1981;178:553–559. [Google Scholar]
- Wright P.J, Neat F.C, Gibb F.M, Gibb I.M, Thordarson H. Evidence for metapopulation structuring in cod from the west of Scotland and North Sea. J. Fish Biol. 2006;69(Suppl. C):181–199. doi:10.1111/j.1095-8649.2006.01262.x [Google Scholar]
- Yoneda M, Wright P.J. Temporal and spatial variation in reproductive investment of Atlantic cod Gadus morhua in the northern North sea and Scottish west coast. Mar. Ecol. Prog. Ser. 2004;276:237–248. doi:10.3354/meps276237 [Google Scholar]