Abstract
The rise of drug resistance remains a major impediment to the treatment of some diseases caused by fast-evolving pathogens that undergo genetic mutations. Models describing the within-host infectious dynamics suggest that the resistance is unlikely to emerge if the pathogen-specific immune responses are maintained above a certain threshold during therapy. However, emergence of resistance in the population involves both within-host and between-host infection mechanisms. Here, we employ a mathematical model to identify an effective treatment strategy for the management of drug resistance in the population. We show that, in the absence of pre-existing immunity, the population-wide spread of drug-resistant pathogen strains can be averted if a sizable portion of susceptible hosts is depleted before drugs are used on a large scale. The findings, based on simulations for influenza infection as a case study, suggest that the initial prevalence of the drug-sensitive strain under low pressure of drugs, followed by a timely implementation of intensive treatment, can minimize the total number of infections while preventing outbreaks of drug-resistant infections.
Keywords: pathogen evolution, treatment, drug resistance, epidemic models
1. Introduction
The development of drug resistance is one of the most challenging public health problems in the treatment of some infectious diseases. This has been well recognized in diseases caused by multi-strain viruses that evolve rapidly and can persist (such as HIV) or infect recurrently (such as influenza; Richman 1994, 1996; Coffin 1995, 1996; Stilianakis et al. 1998; Blower et al. 2003, 2004; Kiso et al. 2004; Bright et al. 2005; de Jong et al. 2005; Moscona 2005), as well as in several bacterial infections (Bonhoeffer et al. 1997; Blower & Chou 2004). Although emergence of resistance involves within-host infectious processes, its intrusive effects often extend beyond the individuals through the transmission of resistant strains in the population. The strategic use of drugs is therefore crucial for preventing population-wide spread of drug resistance, particularly when confronted with the emergence of novel infectious agents (e.g. influenza pandemic viruses), against which the population has little or no pre-existing immunity, effective vaccines may not be available and other intervention measures have limited impact on disease containment.
The initial emergence of resistance is generally associated with a large fitness cost, and therefore resistant strains are soon out-competed if the replication of the drug-sensitive strain is not inhibited (Ferguson et al. 2003). However, treatment can induce a massive selective pressure under which resistant strains may enjoy a growth advantage and restore their impaired fitness (through compensatory mutations) to levels required for successful transmission (Rimmelzwaan et al. 2005; Handel et al. 2006; Regoes & Bonhoeffer 2006). A major barrier to this growth is the generation of an adaptive immune response as a result of interaction between the pathogen and the host immune system. It has been shown that drug resistance is less likely to develop if the immune responses are maintained above a certain threshold during therapy (Wodarz 2001; Wodarz & Lloyd 2004; Lloyd & Wodarz 2006). This suggests that if treatment is initiated after the time at which the immune responses reach the threshold level, the immunity (most profoundly conferred by the formation of cytotoxic T-lymphocytes that destroy infected cells) can impede the replication of resistant strains (Wodarz 2001). However, the development of adaptive immune responses is a time-dependent process (Swain et al. 2004), and late therapy has been shown to be ineffective in the management of self-limiting diseases caused by fast-replicating pathogens, such as influenza (Nelson et al. 2004). Recent clinical observations of kinetics of influenza A infection in humans reveal that these viruses rapidly replicate to high levels of viral titres and deplete the pool of target cells before an effective immune response can be launched (Baccam et al. 2006). While early treatment (within 48 hours of the onset of clinical symptoms) appears to be critical in influenza infection control (Aoki et al. 2003), it poses a major concern for the emergence and spread of drug resistance in the population that should be addressed by assessing the probable epidemiological outcomes of different treatment strategies.
This study undertakes to evaluate the potential benefits and limitations of various treatment strategies on containment of an invading pathogen capable of generating resistance. By employing a population dynamical model, we discuss the influence of three parameters on the spread of drug resistance, namely: (i) the reproduction number of the sensitive strain, (ii) the transmission fitness of the resistant strain, and (iii) the fraction of infected individuals which is treated, referred to as ‘treatment level’. We first consider the scenario in which treatment level is maintained constant during the entire course of an outbreak, and show the existence of an ‘optimal level’ at which the total number of infections is minimum. By allowing treatment level to change during the outbreak, we then project its epidemiological outcomes and demonstrate how insights from within-host immune dynamics can help develop effective strategies for the management of drug resistance in the population. To illustrate the model predictions, parameter estimates of influenza infection are extracted from recent modelling and clinical studies. We organize this paper by developing the model, performing simulations and presenting the results, and finally discussing the significance of our findings.
2. Material and methods
We considered a population that is entirely susceptible to the invading pathogen with no pre-existing immunity. To incorporate pathogen evolution, we extended the classical susceptible–infected–recovered model to include two strains of the pathogen that are sensitive and resistant to the drugs. In the absence of treatment, emergence of drug resistance is unlikely, due to the much lower replicative fitness of resistant mutants compared with the original sensitive strain. We therefore considered the scenario in which drug resistance may develop during the treatment of infected individuals. We assumed that the treatment has no effect in reducing the level or duration of infectiousness in resistant cases. Furthermore, it is assumed that infection caused by the resistant or sensitive strain results in the generation of immunity against both strains upon recovery. These assumptions are valid for a number of infectious pathogens, and we considered influenza infection as a case study. A common situation with influenza is the appearance of two viral strains competing for a given host population (Newman 2005), which often results in dominance by one strain or the development of cross-immunity (Andreasen et al. 1997; Boni et al. 2006). Such competition may even be more complex, particularly when the competing strains enter the population at different times, and we considered this scenario for a resistant strain that appears during the outbreak of the sensitive strain.
(a) The model
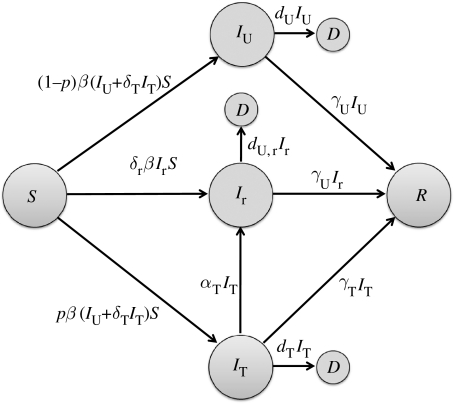
We divided a homogeneously mixing population into several compartments comprising susceptible (S), untreated and treated infected with sensitive strain (IU, IT), infected with resistant strain (Ir), recovered (R) and dead (D) individuals (figure 1). Assuming that the duration of the disease outbreak is short compared with the average lifetime, we ignored the effect of birth and natural death rates on transmission dynamics of infection. Taking into account the above assumptions, the model can be expressed as the following system of deterministic equations:
| (2.1) |
| (2.2) |
| (2.3) |
| (2.4) |
| (2.5) |
| (2.6) |
where the prime ‘′’ denotes the derivative of the compartments with respect to the time; β is the baseline transmission rate of the sensitive strain; δT is the relative infectiousness of treated individuals infected with the sensitive strain; δr represents the relative transmission fitness of the resistant strain; dU and dU,r are disease-induced death rates of sensitive and resistant strains, respectively; γU and γT represent recovery rates of untreated and treated infected individuals, respectively; αT is the rate at which treated individuals develop drug resistance; and p is the fraction of infected individuals who receive treatment (treatment level). Note that since treatment is ineffective against resistant infection, we combined treated and untreated individuals infected with the resistant strain into a single compartment Ir.
Figure 1.
Model diagram for the movement of individuals between population compartments, and the development of drug resistance during treatment of infected individuals.
(b) Reproduction numbers
A key descriptor in determining whether the emerging pathogen can cause a disease outbreak in a population is the basic reproduction number, defined as the number of secondary infections generated by the introduction of a single infected case into an entirely susceptible population (Anderson & May 1992). This number is the product of three parameters: the number of contacts of an infected case with susceptible individuals per unit time; the probability of pathogen transmission; and the generation time. A related quantity is the control reproduction number (Rc) that can be used to evaluate the impact of intervention strategies on containment of disease spread.
To compute Rc in the model (2.1)–(2.6) when treatment is administered, we first assumed that an individual infected with the sensitive strain is introduced into the population of size S0, such that IU(0)=1 and IT(0)=Ir(0)=R(0)=D(0)=0. With the probability p of receiving treatment that reduces the infectiousness (and therefore the transmission of the sensitive strain), the number of new infections emanating from the infected individual during treatment is . Without treatment, the number of secondary cases is given by , and therefore the total number of sensitive infections is
| (2.7) |
An infected individual may develop drug resistance during treatment with probability , and generate a number of new resistant cases given by
| (2.8) |
We now consider the introduction of an infected case with the resistant strain into the population, such that Ir(0)=1 and . Since the generation of new sensitive infections is unlikely, the total number of new cases infected with the resistant strain is given by
| (2.9) |
Considering the generation matrix
the control reproduction number can be obtained by the dominant eigenvalue of G, expressed as (Diekmann & Heesterbeek 2000). In the absence of treatment (p=0), Rc reduces to the basic reproduction number of the sensitive strain, given by .
3. Simulations and results
To illustrate the competitive dynamics between the sensitive and resistant strains, we considered a susceptible population of size S0=100 000 and introduced an initial infection with the sensitive strain (IU(0)=1). Using parameter values estimated for influenza infection given in table 1, we first ran the simulations with low and high relative transmission fitness of the resistant strain, when treatment level p was maintained constant during the entire course of the outbreak.
Table 1.
Parameter values obtained from the published literature for performing numerical simulations of the model (Ferguson et al. 2003, 2005; Longini et al. 2005; Halloran et al. 2006; Handel et al. 2006; Regoes & Bonhoeffer 2006).
| parameter | value (range)a |
|---|---|
| >1 | |
| β | variable (day people)−1 |
| p | 0–1 |
| δT | 0.4 |
| δr | 0–1 |
| αT | 10−6–10−1 d−1 |
| γU | 1/4.1 d−1 |
| γT | 1/4.1 d−1 |
| dU | 0.002 d−1 |
| dT | 0.0002 d−1 |
| dU,r | ∼δrdU d−1 |
For a given value of the basic reproduction number , the transmission rate β can be obtained by substituting parameter values into the expression .
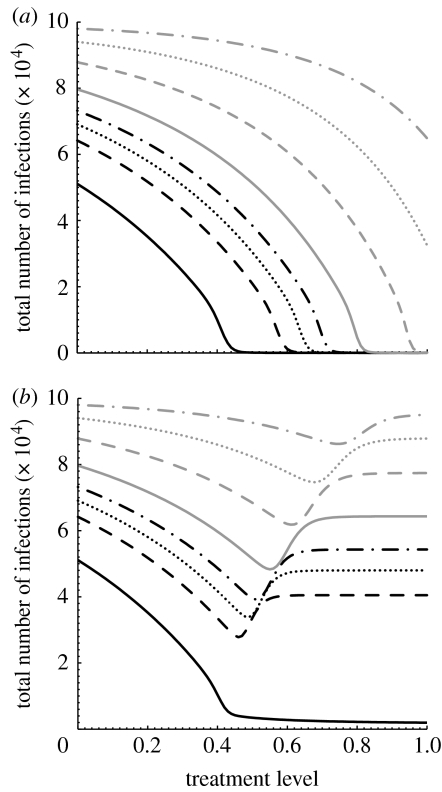
Assuming that the resistant strain emerges with a low transmission fitness δr=0.2, figure 2a shows the total number of infections caused by both strains at the end of the epidemic (including recovered and dead individuals), corresponding to the values of in the range 1.4–4. Since the resistant strain is present at significantly lower transmissibility compared with that of the sensitive strain, a limited number of resistant cases is generated during therapy without promoting the spread of drug resistance, even when the selective pressure of drugs is greatest at high treatment levels. In this case, increasing p would continue to reduce the spread of sensitive strain and disease containment can be achieved if the treatment level results in . Taking into account the reproduction number in equation (2.7), one can easily calculate the critical value p* at which , and therefore the spread of the sensitive pathogen strain can be contained for p>p*. Rewriting in terms of , the value p* is given by
| (3.1) |
However, the spread of disease caused by the sensitive strain cannot be controlled if exceeds
| (3.2) |
which results in p*>1. For the parameter values used in simulations (table 1), this becomes a possibility for a sensitive strain with (figure 2a).
Figure 2.
Total number of infections (final size of the outbreak) caused by both sensitive and resistant strains as a function of treatment level (p) with (a) δr=0.2 and (b) δr=0.8, for different reproduction numbers of the sensitive strain (: black solid line, 1.4; black dashed line, 1.6; black dotted line, 1.7; black dot-dashed line, 1.8; grey solid line, 2; grey dashed line, 2.4; grey dotted line, 3; grey dot-dashed line, 4). Transmission rate β is computed for each value of , and the rate at which infected individuals develop drug resistance is taken to be αT=10−5 d−1. Other parameter values are given in table 1. (a) For below approximately 2.5, the sensitive strain will go extinct if the treatment level exceeds its corresponding threshold (p*), and the disease can be contained for low transmission fitness of the resistant strain. (b) However, for sufficiently high transmission fitness of the resistant strain, high treatment levels may lead to widespread drug resistance.
If the resistant strain emerges with a high transmission fitness or undergoes compensatory mutations to alleviate some of the fitness cost imposed at the initial point of emergence (Rimmelzwaan et al. 2005; Handel et al. 2006), then dynamical interference between competitive strains is more complex. Figure 2b illustrates such complexity for different values of , with δr=0.8. Since treatment reduces the reproduction number of the sensitive strain, the resistant strain may gain a competitive advantage to invade the susceptible hosts, thereby establishing a self-sustaining epidemic. The invasion of the resistant strain (without being out-competed by the sensitive strain) requires , which occurs when the level of treatment exceeds the threshold pc (at which ), given by
| (3.3) |
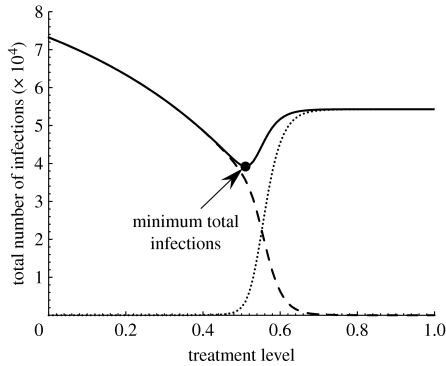
For simulations presented in figure 2b, pc≈0.33. In this case (with a finite susceptible population), a large resistant outbreak is feasible only if the initial propagation of the sensitive strain can be overcome by the selective advantage of the resistant strain before the pool of susceptible hosts is exhausted. Such a scenario can occur for high treatment levels that substantially reduce the spread of the drug-sensitive infection in the population, thereby providing an opportunity for the resistant strain to out-compete the sensitive strain over available susceptible hosts. For , but sufficiently close to 1, the spread of drug resistance is still limited and increasing p reduces the total number of infections (figure 2b). This reduction becomes, however, marginal for higher treatment levels as increases. Simulation results indicate that there is an optimal treatment level associated with that minimizes the total number of infections. However, treatment beyond the optimal level leads to widespread drug resistance by shifting the competitive balance in favour of the resistant strain, and meeting the conditions required for the occurrence of a large resistant outbreak. Figure 2b also shows that the optimal value of p increases with , and the effect of treatment becomes less pronounced in reducing transmission of the sensitive strain. To summarize these results, we numerically calculated the optimal treatment levels for different values of , as illustrated in figure 3. This figure shows that there is a threshold value of (≈1.45), below which the outbreak of both strains can be prevented if all infected individuals are treated (shaded area). However, for values of exceeding the threshold (white area), the fitness advantage of the sensitive strain is overturned by increasing treatment from below to above its optimal level. This can result in a large number of resistant infections that may receive treatment without being effective in the control of disease, as shown in figure 4 for a particular value .
Figure 3.
Optimal treatment level as a function of with δr=0.8 and αT=10−5 d−1. Other parameter values are given in table 1. The shaded area corresponds to the values of below the threshold, and large outbreaks of both sensitive and resistant strains can be prevented with 100% treatment level. In the white area, corresponding to the values of exceeding the threshold, the final size of disease outbreak is minimum if the treatment level is maintained at the optimal level shown by filled circles. The corresponding minimum number of infections is represented by open circles. The ranges of treatment level and minimum number of infections are displayed on the left and right vertical axes, respectively.
Figure 4.
Total number of infections caused by the sensitive (dashed curve), resistant (dotted curve) and both strains (solid curve), as a function of a constant treatment level during the entire course of outbreak, with , δr=0.8 and αT=10−5 d−1. Other parameters are taken from table 1. Total number of infections (including recovered and dead individuals) is minimum at 51% treatment level, and the spread of the sensitive strain is contained at 75% treatment level. Note that the total number of resistant infections include both the emergence of resistance during treatment and through the direct transmission of the resistant strain.
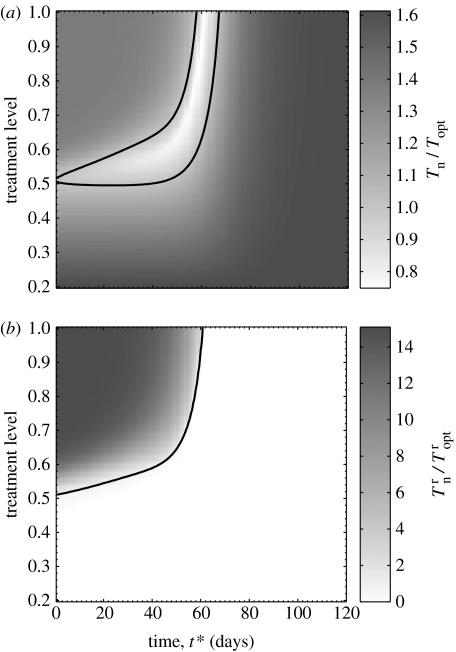
For comparison purposes, we investigated an alternative strategy that allows for changing p at a specified time during the outbreak. We assumed that and defined two quantities: (i) the total number of infections (Tn) as a function of p and the time t* at which the initial treatment level changes, and (ii) the total number of infections (Topt) when treatment is maintained constant at the optimal level (which is 51% for ), as discussed above. Simulations were run with an initial 20% treatment level to compute the ratio Tn/Topt. Figure 5a shows that increasing p at a later stage during the outbreak can reduce the final size of infections significantly below the minimum achieved at a 51% constant treatment level (i.e. Tn/Topt<1). However, aggressive treatment early on (during the seed phase of the disease) leads to widespread drug resistance, and therefore increases the final size of infections. On the other hand, the sensitive strain depletes the pool of susceptible hosts if treatment is administered at a low level or increased to above the optimal level during the late stage of the outbreak (figure 5a). Therefore, a timely increase in the treatment level is critically important in reducing the overall infections when treatment is initiated at a low level.
Figure 5.
The effect of changing treatment level during the outbreak on the total number of infections (including recovered and dead cases) generated through the transmission of sensitive and resistant strains, with , δr=0.8 and αT=10−5 d−1. Other parameter values are given in table 1. Simulations were seeded with an initial treatment level of 20% and then changed to the level shown on the vertical axis at the time t* displayed on the horizontal axis (corresponding to the time course of the outbreak). The grey bar in (a) illustrates the ratio of the total number of infections caused by both strains Tn to that generated when treatment is maintained constant at the optimal level of 51%. The grey bar in (b) shows the ratio of the total number of resistant infections to that generated when treatment is maintained constant at the optimal level. The curves in (a,b) correspond to the Tn=Topt=1 and isoclines, respectively.
To explore the impact of this strategy on the spread of drug resistance, we performed further simulations to compute the ratio , where and denote the corresponding total number of resistant infections. The results, displayed in figure 5b, suggest that the population-wide spread of drug resistance can be averted for certain combinations of p and t*, resulting in Tn=31 335 and when p is increased to 80% at time t*=60 days. This, compared with the corresponding numbers Topt=39 203 and obtained at a 51% constant treatment strategy, shows that a significant (20%) reduction in the final size of infections can be achieved, while preventing large outbreaks of resistant cases ().
These simulations show that if an initially high treatment level fails to contain the disease, then large outbreaks of resistant infections can occur (figure 5). There are two major contributing factors that promote the emergence of resistance in the population. First, intensive treatment can exert a strong selective pressure under which the resistant strain replicates rapidly in individuals and spreads easily between them before the pool of susceptible hosts is depleted. Second, large-scale drug use at the early stages of an outbreak decelerates the spread of the sensitive strain, and therefore largely interferes with the rise of population immunity as a protective mechanism against the generation of pathogen strains. These factors correspond to those that are most responsible for the emergence of drug resistance in vivo (in the absence of immunological control of infection), as discussed in previous studies (Wodarz 2001; Wodarz & Lloyd 2004; Lloyd & Wodarz 2006).
4. Discussion
Drug resistance remains a major obstacle to the management of some persistent pathogens (e.g. HIV, influenza, tuberculosis) and limits our ability to effectively respond to the emergence of novel infectious agents (e.g. an influenza pandemic). Several studies have discussed factors promoting the evolution of drug resistance in vivo, and highlighted the importance of an effective immune response in preventing the outgrowth of resistant strains (Wodarz 2001; Wodarz & Lloyd 2004; Lloyd & Wodarz 2006). However, a different scenario occurs when a drug-resistant strain is transmitted to a susceptible individual who lacks pre-existing pathogen-specific immunity. In the absence of a sensitive strain as the dominant competitor, the resistant strain will replicate to generate well-adapted mutants in the new drug-free environment. The effective rate of adaptation is determined not only by the rate at which mutations occur but also by the fitness of generated mutants in multiple hosts (Kuiken et al. 2006), which is generally accompanied by an increase in fitness. To minimize the likelihood of resistance invasion in a population, it is therefore essential to halt the transmission of resistant strains between susceptible hosts.
In this paper, we developed a two-strain population dynamical model to evaluate the epidemiological outcomes of different therapeutic strategies with constant and variable treatment levels in the presence of drug resistance. We focused on the scenario in which a resistant strain emerges with transmission fitness comparable with that of the sensitive strain, since drug-resistant strains with low transmissibility are unlikely to grow and are soon out-competed. For the strategy with a constant treatment level throughout an outbreak, we have shown that there is an optimal level at which the final size of infections is minimum. While raising the treatment level beyond its optimal level further reduces the transmission of the sensitive strain, it can potentially offset the effectiveness of drugs by increasing the size of the outbreak with the resistant strain (figure 2b).
To prevent the spread of resistance in the population, we considered an alternative strategy that delays the application of intensive treatment for a certain amount of time after the onset of an outbreak. Since immunity induced by natural infection will protect individuals against all pathogen strains, the time at which selection of resistance occurs during the outbreak of the sensitive strain will be crucial. If a significant number of susceptible hosts have already been infected with the sensitive strain, the selective advantage of the resistant strain may not be enough for a subsequent resistant outbreak. This suggests that, in the absence of pre-existing immunity, the initial propagation of the sensitive strain under low pressure of drugs can reduce the likelihood of resistant outbreaks occurring. To investigate this scenario, we allowed the treatment level to change during the outbreak and observed that, under certain conditions, the spread of resistant strains can be prevented, while minimizing the overall number of infections. However, the impact of this strategy depends critically on the initial treatment level and its timely increase so that the transmission of the sensitive strain can be effectively blocked. For simulations presented in figure 5, the susceptibility of the population at time t*=60 (days) is reduced by approximately 13%, which is sufficient to rule out the possibility of a resistant outbreak when the treatment level is increased to 80%. Obviously, the time t* varies with parameters and the number of infected cases at the onset of the outbreak (in the absence of resistance), as well as the initial scale of drug use. Higher numbers of initial infections can result in a more rapid spread of the sensitive pathogen strain in the population, and consequently an earlier increase in the level of treatment is required. On the other hand, as the initial treatment level increases, a greater reduction in transmission of the sensitive strain is achieved, and therefore the onset of intensive treatment should be further delayed.
The findings of this study have important implications for the strategic use of drugs in a population, especially in response to the emergence of novel infectious pathogens. There is mounting concern for an imminent influenza pandemic due to outbreaks of a highly pathogenic avian strain (H5N1) in poultry (Gani et al. 2005; Jennings & Peiris 2006), which has resulted in a large mortality associated with indexed human cases (WHO, http://www.who.int/csr/disease/avian_influenza/cases_table_2007_10_02/en/index.html). In the absence of effective vaccines, antiviral drugs have been rationalized as the primary measure in mitigating the impact of the next influenza pandemic (Ferguson et al. 2005; Gani et al. 2005; Longini et al. 2005). However, the identification of resistance to neuraminidase inhibitors (de Jong et al. 2005; Moscona 2005; Yen et al. 2005) poses a major challenge to the prudent use of drugs when the early treatment of indexed cases is crucial for reducing viral transmission (Alexander et al. 2007, 2008). Our results in this study indicate that if the pandemic virus is not contained at the source, then intensive treatment early on during the first wave of infection could potentially lead to a worldwide pandemic of resistant viral strains. Therefore, the risk of drug resistance should be considered in designing antiviral strategies, in order to not only maximize the population-wide benefit of drugs, but also preserve the capacity to cope with surging demand in treatment with a limited drug supply.
The modelling efforts in this study aimed to project the probable patterns of disease spread in a population for identifying effective treatment strategies in the context of drug resistance. The work is based on a two-strain compartmental model with relatively few parameters, under the assumption of homogeneity in population interactions. Such models offer a simple approach to the general understanding of determinants in the course of an outbreak and the impact of interventions (Anderson 2006). There is clearly a need for further investigation of treatment strategies when heterogeneity of the population with detailed structure of network contacts and mobility patterns, as well as stochastic effects on the initial emergence of resistance (with low rates of resistant mutations), are taken into account (Handel et al. 2006, 2007; Débarre et al. 2007). We based our results on numerical simulations for influenza infection spread using estimated parameters from the published literature. Although model simulations may be quantitatively altered by the values of related parameters, we emphasize the qualitative aspects of the findings that can be applied to the design of treatment measures against other invading pathogens with similar infectious dynamics. The practical implementation of the proposed strategy requires a rapid identification of the transmission characteristics of the pathogen for determining its reproduction number. This should be integrated with surveillance and monitoring systems during the course of an outbreak to identify the emergence and transmission fitness of resistance, so that necessary adaptations to the treatment strategy can be made in a timely fashion.
Acknowledgments
This research was in part supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and Mathematics of Information Technology and Complex Systems (MITACS). The author would like to thank the reviewers for their insightful comments that have greatly improved this paper.
References
- Alexander M.E, Bowman C.S, Feng Z, Gardam M, Moghadas S.M, Röst G, Wu J, Yan P. Emergence of drug-resistance: implications for antiviral control of pandemic influenza. Proc. R. Soc. B. 2007;274:1675–1684. doi: 10.1098/rspb.2007.0422. doi:10.1098/rspb.2007.0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M.E, Moghadas S.M, Röst G, Wu J. A delay differential model for pandemic influenza with antiviral treatment. Bull. Math. Biol. 2008;70:382–397. doi: 10.1007/s11538-007-9257-2. doi:10.1007/s11538-007-9257-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.M. Planning for pandemics of infectious diseases. Bridge. 2006;36:5–9. [Google Scholar]
- Anderson R.M, May R.M. Oxford University Press; Oxford, UK: 1992. Infectious diseases of humans. [Google Scholar]
- Andreasen V, Lin J, Levin S.A. The dynamics of cocirculating influenza strains conferring partial cross-immunity. J. Math. Biol. 1997;35:825–842. doi: 10.1007/s002850050079. doi:10.1007/s002850050079 [DOI] [PubMed] [Google Scholar]
- Aoki F.Y, et al. Early administration of oral oseltamivir increases the benefits of influenza treatment. J. Antimicrob. Chemother. 2003;51:123–129. doi: 10.1093/jac/dkg007. doi:10.1093/jac/dkg007 [DOI] [PubMed] [Google Scholar]
- Baccam P, Beauchemin C, Macken C.A, Hayden F.G, Perelson A.S. Kinetics of influenza A virus infection in humans. J. Virol. 2006;80:7590–7599. doi: 10.1128/JVI.01623-05. doi:10.1128/JVI.01623-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower S, Chou T. Modeling the emergence of the ‘host zones’: tuberculosis and the amplification dynamics of drug-resistance. Nat. Med. 2004;10:1111–1116. doi: 10.1038/nm1102. doi:10.1038/nm1102 [DOI] [PubMed] [Google Scholar]
- Blower S, Ma L, Farmer P, Koenig S. Predicting the impact of antiretrovirals in resourse-poor settings: preventing HIV infection whilst controlling drug resistance. Curr. Drugs Targets Infect. Disord. 2003;3:345–353. doi: 10.2174/1568005033480999. doi:10.2174/1568005033480999 [DOI] [PubMed] [Google Scholar]
- Blower S, Bodine E, Khan J, McFarland W. The antiretroviral rollout and drug-resistant HIV in Africa: insights from empirical data and theoretical models. AIDS. 2004;19:1–14. doi: 10.1097/00002030-200501030-00001. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer S, Lipsitch M, Levin B.R. Evaluating treatment protocols to prevent antibiotic resistance. Proc. Natl Acad. Sci. USA. 1997;94:12 106–12 111. doi: 10.1073/pnas.94.22.12106. doi:10.1073/pnas.94.22.12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni M.F, Gog J.R, Andreasen V, Feldman M.W. Epidemic dynamics and antigenic evolution in a single season of influenza A. Proc. R. Soc. B. 2006;273:1307–1316. doi: 10.1098/rspb.2006.3466. doi:10.1098/rspb.2006.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R.A, Medina M.J, Xu X, Perez-Oronoz G, Wallis T.R, Davis X.M, Povinelli L, Cox N.J, Klimov A.I. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 2005;366:1175–1181. doi: 10.1016/S0140-6736(05)67338-2. doi:10.1016/S0140-6736(05)67338-2 [DOI] [PubMed] [Google Scholar]
- Coffin J.M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. doi:10.1126/science.7824947 [DOI] [PubMed] [Google Scholar]
- Coffin J.M. HIV viral dynamics. AIDS. 1996;10(Suppl. 3):S75–S84. [PubMed] [Google Scholar]
- Débarre F, Bonhoeffer S, Regoes R.R. The effect of population structure on the emergence of drug-resistance during pandemic influenza. J. R. Soc. Interface. 2007;4:893–906. doi: 10.1098/rsif.2007.1126. doi:10.1098/rsif.2007.1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong M.D, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. NEJM. 2005;353:2667–2672. doi: 10.1056/NEJMoa054512. doi:10.1056/NEJMoa054512 [DOI] [PubMed] [Google Scholar]
- Diekmann O, Heesterbeek J.A.P. Wiley; Chichester, UK: 2000. Mathematical epidemiology of infectious diseases. [Google Scholar]
- Ferguson N.M, Mallett S, Jackson H, Roberts N, Ward P. A population-dynamic model for evaluating the potential spread of drug-resistant influenza virus infections during community-based use of antivirals. J. Antimicrob. Chemother. 2003;51:977–990. doi: 10.1093/jac/dkg136. doi:10.1093/jac/dkg136 [DOI] [PubMed] [Google Scholar]
- Ferguson N.M, Cummings D.A.T, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke D.S. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–214. doi: 10.1038/nature04017. doi:10.1038/nature04017 [DOI] [PubMed] [Google Scholar]
- Gani R, Hughes H, Fleming D, Griffin T, Medlock J, Leach S. Potential impact of antiviral drug use during influenza pandemic. Emerg. Infect. Dis. 2005;9:1355–1362. doi: 10.3201/eid1109.041344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran M.E, Hayden F.G, Yang Y, Longini I.M, Jr, Monto A.S. Antiviral effects on influenza viral transmission and pathogenicity: observations from household-based trials. Am. J. Epidemiol. 2006;165:212–221. doi: 10.1093/aje/kwj362. doi:10.1093/aje/kwj362 [DOI] [PubMed] [Google Scholar]
- Handel A, Regoes R.R, Antia R. The role of compensatory mutations in the emergence of drug resistance. PLoS. 2006;2:1262–1270. doi: 10.1371/journal.pcbi.0020137. doi:10.1371/journal.pcbi.0020137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel A, Longini I.M, Jr, Antia R. Neuraminidase inhibitor resistance in influenza: assessing the danger of its generation and spread. PLoS Comput. Biol. 2007;3:e240. doi: 10.1371/journal.pcbi.0030240. doi:10.1371/journal.pcbi.0030240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings L.C, Peiris M. Avian influenza H5N1: is it a cause for concern? Intern. Med. J. 2006;36:145–147. doi: 10.1111/j.1445-5994.2006.01036.x. doi:10.1111/j.1445-5994.2006.01036.x [DOI] [PubMed] [Google Scholar]
- Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, Hayden F.G, Sugaya N, Kawaoka Y. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759–765. doi: 10.1016/S0140-6736(04)16934-1. doi:10.1016/S0140-6736(04)16934-1 [DOI] [PubMed] [Google Scholar]
- Kuiken T, Holmes E.C, McCauley J, Rimmelzwaan G.F, Williams C.S, Grenfell B.T. Host species barriers to influenza virus infections. Science. 2006;312:394–397. doi: 10.1126/science.1122818. doi:10.1126/science.1122818 [DOI] [PubMed] [Google Scholar]
- Lloyd, A. L. & Wodarz, D. 2006 Drug-resistance in acute viral infections: rhinovirus as a case study. In Disease evolution: models, concepts, and data analyses DIMACS series in discrete mathematics and theoretical computer science, pp. 193–212. Providence, RI: AMS.
- Longini I.M, Jr, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings D.A.T, Halloran M.E. Containing pandemic influenza at the source. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. doi:10.1126/science.1115717 [DOI] [PubMed] [Google Scholar]
- Moscona A. Oseltamivir resistance—disabling our influenza defenses. N. Engl. J. Med. 2005;353:2633–2636. doi: 10.1056/NEJMp058291. doi:10.1056/NEJMp058291 [DOI] [PubMed] [Google Scholar]
- Nelson K.E, Williams C.M, Graham N.M.H. Jones and Bartlett Publishers; Sudbury, MA: 2004. Infectious disease epidemiology, theory and practice. [Google Scholar]
- Newman M.E.J. Threshold effects for two pathogens spreading on a network. Phys. Rev. Lett. 2005;95:108 701. doi: 10.1103/PhysRevLett.95.108701. doi:10.1103/PhysRevLett.95.108701 [DOI] [PubMed] [Google Scholar]
- Regoes R.R, Bonhoeffer S. Emergence of drug-resistance influenza virus: population dynamical considerations. Science. 2006;312:389–391. doi: 10.1126/science.1122947. doi:10.1126/science.1122947 [DOI] [PubMed] [Google Scholar]
- Richman D.D. Drug resistance in viruses. Trends Microbiol. 1994;2:401–407. doi: 10.1016/0966-842x(94)90619-x. doi:10.1016/0966-842X(94)90619-X [DOI] [PubMed] [Google Scholar]
- Richman D.D. Antiretroviral drug resistance: mechanisms, pathogenesis, clinical significance. Adv. Exp. Med. Biol. 1996;394:383–395. doi: 10.1007/978-1-4757-9209-6_35. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan G.F, Berkhoff E.G.M, Nieuwkoop N.J, Smith D.J, Fouchier R.A.M, Osterhaus A.D.M.E. Full restoration of viral fitness by multiple compensatory co-mutations in the nucleoprotein of influenza A virus cytotoxic T-lymphocyte escape mutants. J. Gen. Virol. 2005;86:1801–1805. doi: 10.1099/vir.0.80867-0. doi:10.1099/vir.0.80867-0 [DOI] [PubMed] [Google Scholar]
- Stilianakis N.I, Perelson A.S, Hayden F.G. Emergence of drug resistance during an influenza epidemic: insights from a mathematical model. J. Infect. Dis. 1998;177:863–873. doi: 10.1086/515246. [DOI] [PubMed] [Google Scholar]
- Swain S.L, Dutton R.W, Woodland D.L. T cell responses to influenza virus infection: effector and memory cells. Viral. Immunol. 2004;17:197–209. doi: 10.1089/0882824041310577. doi:10.1089/0882824041310577 [DOI] [PubMed] [Google Scholar]
- Wodarz D. Helper-dependent vs. helper-independent CTL responses in HIV infection: implications for drug therapy and resistance. J. Theor. Biol. 2001;213:447–459. doi: 10.1006/jtbi.2001.2426. doi:10.1006/jtbi.2001.2426 [DOI] [PubMed] [Google Scholar]
- Wodarz D, Lloyd A.L. Immune responses and the emergence of drug-resistant virus strains in vivo. Proc. R. Soc. B. 2004;271:1101–1109. doi: 10.1098/rspb.2003.2664. doi:10.1098/rspb.2003.2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H.L, Herlocher L.M, Hoffmann E, Matrosovich M.N, Monto A.S, Webster R.G, Govorkova E.A. Neuraminidase inhibitor-resistant influenza viruses may differ substantially in fitness and transmissibility. Antimicrob. Agents Chemother. 2005;49:4075–4084. doi: 10.1128/AAC.49.10.4075-4084.2005. doi:10.1128/AAC.49.10.4075-4084.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]