Abstract
Vinblastine and other microtubule inhibitors used as antimitotic cancer drugs characteristically promote the phosphorylation of the key anti-apoptotic protein, Bcl-xL. However, putative sites of phosphorylation have been inferred based on potential recognition by JNK, and no direct biochemical analysis has been performed. In this study we used protein purification and mass spectrometry to identify Ser-62 as a single major site in vivo. Site-directed mutagenesis confirmed Ser-62 to be the site of Bcl-xL phosphorylation induced by several microtubule inhibitors tested. Vinblastine-treated cells overexpressing a Ser-62 → Ala mutant showed highly significantly reduced apoptosis compared with cells expressing wild-type Bcl-xL. Co-immunoprecipitation revealed that phosphorylation caused wild-type Bcl-xL to release bound Bax, whereas phospho-defective Bcl-xL retained the ability to bind Bax. In contrast, phospho-mimic (Ser-62 → Asp) Bcl-xL exhibited a reduced capacity to bind Bax. Functional tests were performed by transiently co-transfecting Bax in the context of different Bcl-xL mutants. Co-expression of wild-type or phospho-defective Bcl-xL counteracted the adverse effects of Bax expression on cell viability, whereas phospho-mimic Bcl-xL failed to provide the same level of protection against Bax. These studies suggest that Bcl-xL phosphorylation induced by microtubule inhibitors plays a key pro-apoptotic role at least in part by disabling the ability of Bcl-xL to bind Bax.
Mitochondrial or intrinsic apoptosis is regulated by the Bcl-2 family of proteins which exhibit either pro- or anti-apoptotic properties (for review, see Refs. 1–3). The BH3-only pro-apoptotic members act as essential initiators of intrinsic apoptosis, whereas the multidomain pro-apoptotic Bax and Bak act as essential mediators of mitochondrial membrane permeability. Bcl-2 proteins function in a hierarchical fashion, and different models have been proposed to explain their complex interrelationships (1–3). The activator-sensitizer model proposes that BH3-only proteins comprise two subsets, the activators and the sensitizers. Activators, which include Bim and Bid, once activated themselves, in turn directly activate Bax and Bak. Sensitizers such as Bad and Bik, on the other hand, appear to act by neutralizing anti-apoptotic Bcl-2 family members and do so by displacing activator proteins bound to anti-apoptotic Bcl-2 proteins or by precluding anti-apoptotic Bcl-2 proteins from sequestering activator BH3-only proteins. In the indirect model Bax and Bak are bound to anti-apoptotic Bcl-2 proteins and become liberated and activated as a consequence of displacement by BH3-only proteins.
Microtubule inhibitors are a class of compounds that bind to tubulin or microtubules and suppress microtubule dynamics (for review, see Refs. 4–6). Treatment of cells with these agents leads to prolonged activation of the mitotic spindle checkpoint, mitotic arrest, and eventually cell death, providing a rationale for their use as antitumor agents. The first microtubule inhibitor utilized for cancer chemotherapy was the vinca alkaloid vinblastine from the plant Catharanthus roseus, which was put into clinical practice to treat pediatric hematological malignancies in the mid-1950s. Currently, vinblastine is used in adjuvant and combination chemotherapy for several tumor types including breast, testicular, Hodgkin lymphoma, non-Hodgkin lymphoma, and Kaposi sarcoma. Another class of microtubule inhibitors is the taxanes with paclitaxel (Taxol, Food and Drug Administration approval in 1995), becoming a highly successful anticancer agent.
Although microtubule inhibitors have played a key role in cancer chemotherapy for many decades, a molecular explanation of how cells die in response to mitotic checkpoint activation has proven difficult to establish. One of the complicating factors is that microtubule inhibitors activate many different signaling pathways (4, 7). However, one highly consistent observation is the phosphorylation of anti-apoptotic Bcl-2 proteins including Bcl-2 (8) and Bcl-xL (9). Although Bcl-2 appears to undergo different types of phosphorylation involved in both cell survival and cell death, extensive multisite phosphorylation occurs in response to microtubule inhibitors (for review, see Ref. 10). Expression of phospho-defective forms of Bcl-2 renders some cell types more resistant to microtubule inhibitors, suggesting that phosphorylation antagonizes Bcl-2 anti-apoptotic function (11, 12), whereas another study suggested that drug-induced multisite phosphorylation promotes Bcl-2 anti-apoptosis function through protein stabilization (13).
Bcl-xL phosphorylation also occurs in response to microtubule inhibitors (9) and other apoptotic stimuli including ionizing radiation (14). In light of information that, among many apoptotic regulatory proteins, Bcl-xL expression correlates uniquely with drug resistance (15), it is particularly important to understand mechanisms that govern Bcl-xL function. However, information on the functional significance and enzymology of Bcl-xL phosphorylation is controversial. For example, whereas several reports suggest that phosphorylation is catalyzed by c-Jun NH2-terminal kinase (JNK),3 in one study phosphorylation was reported to occur on serine (serine 62) (16), and in another study phosphorylation was reported to occur on threonine residues (threonines 47 and 115) (14). These apparent discrepancies appear to stem from the fact that potential sites of phosphorylation have been inferred based in particular on the assumption that JNK is the responsible kinase rather than directly determined. However, studies implicating JNK in microtubule inhibitor-induced Bcl-xL/Bcl-2 phosphorylation (12, 14, 16) have been complicated by the fact that JNK also plays a key role in cell cycle progression. In a carefully designed study using synchronized cells examined over an extended time-course, we showed that when cells are treated with both a JNK inhibitor and a microtubule inhibitor, the JNK inhibitor slows the normal progression to mitotic arrest, and events such as Bcl-xL/Bcl-2 phosphorylation are delayed by many hours (17). Nonetheless, in the context of validated JNK inhibition, microtubule inhibitor-induced Bcl-xL/Bcl-2 phosphorylation still occurs to a normal extent (17). Thus, conclusions regarding the effect on Bcl-xL/Bcl-2 function of mutation of putative JNK sites are tempered by apparent misidentification of the kinase responsible.
In this study we report the direct biochemical identification of a major site of phosphorylation in Bcl-xL induced by vinblastine and other microtubule inhibitors. This approach represents an unequivocal determination of the authentic site of phosphorylation and is not subject to the uncertainty that can arise when sites are inferred via indirect mutagenesis-based approaches. Site-directed mutagenesis showed that cells expressing a validated phospho-defective Bcl-xL mutant are highly vinblastine-resistant, indicating that phosphorylation is a key regulatory mechanism for antagonizing anti-apoptotic function. Furthermore, co-expression of wild-type or phospho-defective Bcl-xL conferred resistance to Bax induced cell death, whereas a phospho-mimic Bcl-xL mutant failed to protect cells from Bax, in part due to reduced Bax binding. These studies provide mechanistic insight into the role of Bcl-xL phosphorylation induced by microtubule inhibitors.
EXPERIMENTAL PROCEDURES
Materials—Bcl-xL antibody was from Cell Signaling Technology (catalogue number 2762; for immunoprecipitation) or Santa Cruz (catalogue number sc-509, clone H-5; for immunoblotting). Bax antibody (catalogue number 2772) and active caspase-3 antibody (catalogue number 9661) were from Cell Signaling Technology, and active Bax antibody 6A7 was from Axxora. Procaspase-3 antibody (catalogue number sc-7148) was from Santa Cruz, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (catalogue number 4300) antibody was from Ambion, Cox II (Complex IV) antibody was from an OXPHOS complex detection kit (MitoSciences), and anti-rabbit IgG-agarose beads were from eBioscience (catalogue number 00-8800-26). Neomycin (G418 sulfate) was from Mediatech, Mitotracker Red was from Molecular Probes, and fluorescein isothiocyanate-labeled anti-rabbit goat antibody and vinblastine were from Sigma-Aldrich.
Cell Culture and Transfection—The KB-3 cell line was maintained in monolayer culture at 37 °C and 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mm glutamine, 50 unit/ml penicillin, and 50 mg/ml streptomycin. Cells stably overexpressing HA-Bcl-xL or phospho-mutants were prepared by transfecting KB-3 cells with human Bcl-xL tagged with HA epitope in pCDNA3.1 vector and maintained in 0.5 mg/ml G418 as described (18).
Preparation of Cell Extracts and Immunoblotting—Whole cell extracts were prepared and immunoblotted as described (17). Cytosolic and mitochondrial fractions were prepared using the sub-cell fractionation kit from Focus™ (catalogue number 786-260).
Immunofluorescence Staining—Cells were permeabilized with 1% Triton-X100 for 5 min and blocked with 10% goat serum for 20 min. Cells were then incubated with Bcl-xL antibody (Cell Signaling Technology) for 2 h at a dilution of 1:100 and then washed with phosphate-buffered saline and incubated with fluorescein isothiocyanate goat anti-rabbit secondary antibody for 1 h at a dilution of 1:1000. All steps were performed at room temperature. To visualize mitochondria, cells were incubated with Mitotracker Red (1:1000) for 5 min at 37 °C and fixed with 3.7% formaldehyde in phosphate-buffered saline for 30 min. Immunofluorescent protein and mitochondria were visualized using 488 and 568 laser lines of a Zeiss LSM410 confocal microscope.
3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium Bromide (MTT) Cell Viability Assay—Cells (2000/well) were seeded in 96-well plates, and the following day vinblastine was added. After 72 h MTT (50 mg/well) was added for 4 h followed by dimethyl sulfoxide solubilization of the cells, and absorbance was read at 540 nm. Data are expressed relative to untreated controls.
Apoptosis Assay—Untreated and treated cells were diluted to a concentration of 5 × 104 cells/ml for measurement of apoptosis using the cell death detection enzyme-linked immunosorbent assay kit (Roche Applied Science). In this colorimetric assay values are represented by absorbance at 405 nm.
Immunoprecipitation—Optimization of Bcl-xL immunoprecipitation was performed using methodology described previously (19) with slight modifications. Briefly, cells were lysed in 0.3 ml of 40 mm HEPES (pH 7.5), 0.12 m NaCl, 1% Triton X-100, 1 mm EDTA, containing phosphatase and protease inhibitors and, after 30 min on ice, centrifuged at 9200 × g for 20 min. The extract (1.0 mg) was precleared with anti-rabbit goat IgG agarose beads according to the manufacturer's directions (eBiosciences) and to the supernatant was added rabbit polyclonal antibody (2–7.5 μg). After mixing for 2 h, anti-rabbit goat IgG-agarose beads were added and mixed overnight. After centrifugation, the supernatant was recovered, and the immunoprecipitate was washed as described previously (18) and resuspended in SDS sample buffer. Equivalent portions of supernatant and precipitate were subjected to 12.5% acrylamide SDS-PAGE, and immunoblotting which was performed with monoclonal mouse antibodies.
Preparative immunoprecipitation of Bcl-xL was carried out using the same protocol except that extracts containing 8 mg of protein, derived from ∼108 cells, were used for each condition, and the amounts of antibody and IgG beads were increased proportionally. After the immunoprecipitates were washed, elution was performed in 1 ml of 0.5 m NH4OH, 0.5 mm EDTA, and after incubation with rotation for 10 min at room temperature, samples were dried in a speed vacuum overnight. Samples were resolved by 13.5% acrylamide SDS-PAGE using separation gels 15 cm in length and stained in Colloidal Coomassie Blue (Pierce). Examination of the interaction of Bcl-xL and Bax by co-immunoprecipitation was performed as described previously (18).
Mass Spectrometry—Gel bands were excised, and Coomassie was removed by serial washing with 50% methanol, 0.1 m ammonium bicarbonate. Each gel band was divided into two portions of which half was digested with 75 ng of trypsin and the other half with 100 ng of endoproteinase AspN (Roche Applied Science). The resulting peptides were spotted in 2,5-dihydroxybenzoic acid for MALDI-MS and MALDI-MS2 analysis (20, 21). A high accuracy (∼5 ppm) mass spectrum (MALDI-MS) was taken of each sample with a PerkinElmer Life Sciences prOTOF mass spectrometer. Utilizing a custom engineered sample stage, the peptides observed from the spectra collected with the prOTOF mass spectrometer were subjected to tandem mass spectrometric analysis (MALDI-MS2) with a Thermo vMALDI-LTQ ion trap mass spectrometer. A single data file containing the high accuracy peptide measurements and fragmentation information was used to identify Bcl-xL via data base searching with the program XProteo (21). The combination of peptides resulting from trypsin and AspN digestion provided for 64% sequence coverage of Bcl-xL.
Peptide Phosphorylation—A peptide corresponding to residues 58–66 of Bcl-xL containing Ser-62 with 3 C-terminal arginines (HLADSPAVNRRR) and termed FL62 (for flexible loop) was synthesized by GenScript. Phosphorylation reactions contained 5 μg of whole cell extract (17) and 40 μg of FL62 in 25 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 5 mm dithiothreitol, 1 μm ATP, and 1 μCi of [γ-32P]ATP and were incubated for 20 min at 30 °C. Reactions were stopped by the addition of 0.2 m EDTA to 20 mm and boiled in SDS sample buffer, and aliquots containing 10 μg of FL62 were applied to 16.5% acrylamide gels. Gels were fixed overnight in 5% glutaraldehyde, washed with H2O, exposed to a PhosphorImager screen for 48 h, and an image was obtained using a PhosphorImager processed using ImageQuant NT (Amersham Biosciences).
Site-directed Mutagenesis—pHA-Bcl-xL/pcDNA3.1 (plasmid encoding HA-tagged human Bcl-xL in pcDNA3.1 vector) served as a template. The HA-Bcl-xL (S62A), HA-Bcl-xL (T47A), and HA-Bcl-xL (S62D) mutants were generated using the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The nucleotide sequences of all cDNA constructs were verified by automated DNA sequencing.
Transient Transfection—4 μg of pCDNA3.1 or plasmids encoding wild-type HA-Bcl-xL, phospho-mimic (HA-Bcl-xL(S62A)), or phospho-defective (HA-Bcl-xL(S62D)) mutant constructs were used to transfect KB-3 cells using Lipofectamine2000 transfection reagent (Invitrogen) according to the manufacturer's protocol. Co-transfections were performed using 2 μgof the above plasmids plus 2 μg of Myc-CMV or Myc-Bax. 48 h after transfection whole cell extracts were made to assess the level of transfected protein by immunoblotting. To assess cell survival, non-viable (non-adherent) cells were removed and counted, and adherent cells were visualized under phase contrast microscopy, photographed, and then recovered by trypsinization and counted.
Bax Activation—Assessment of Bax activation was performed as described previously (18).
RESULTS
Validation of Bcl-xL-overexpressing Cells as a Model System—The KB-3 cell line undergoes a well defined mitotic arrest followed by apoptotic cell death in response to treatment with vinblastine and is readily transfectable and easy to synchronize (17). Thus, it represents an excellent model system for understanding the molecular link between mitotic arrest and apoptosis and for examining the role of Bcl-xL phosphorylation during these processes. Based on evidence derived from two-dimensional gel electrophoresis that Bcl-xL is a very low abundance protein, we generated KB-3 cells overexpressing HA-tagged Bcl-xL (termed KB-3/HA-Bcl-xL) to increase the amount of protein available for purification (Fig. 1A). Subcellular fractionation indicated that HA-Bcl-xL was primarily mitochondrial and also present in the cytosol (Fig. 1B), as found for the endogenous protein (17) (note that under these immunoblotting conditions, endogenous Bcl-xL is not detectable, but see Fig. 3). That HA-Bcl-xL was mainly mitochondrial was confirmed by immunofluorescent staining (supplemental Fig. S1). Procaspase 3 was used as a cytosolic marker, and it is evident that it was more susceptible to loss in vinblastine-treated KB-3 cells versus KB-3/HA-Bcl-xL cells (Fig. 1B). In concert, active caspase-3 was detectable in KB-3 cells but undetectable in KB-3/HA-Bcl-xL cells after vinblastine treatment (Fig. 1C). More comprehensive studies were performed to characterize the drug-resistant properties of KB-3/HA-Bcl-xL cells. Assays evaluating cell survival and apoptosis demonstrated that cells overexpressing HA-Bcl-xL were markedly vinblastine-resistant (Fig. 2, A and B). Indeed, although 1 nm vinblastine was sufficient to kill the majority of KB-3 cells (IC50 of 0.6 nm), 50% survival was observed for HA-Bcl-xL-overexpressing cells treated with 10–150 nm vinblastine (Fig. 2A). Because these assays measure the number of viable cells relative to untreated cells (which continue to proliferate during this interval), the results are consistent with HA-Bcl-xL-overexpressing cells being susceptible to vinblastine-induced growth arrest but resistant to subsequent cell death. This notion was supported by evaluation of cyclin B levels which confirmed that vinblastine caused mitotic arrest independent of Bcl-xL expression level (see supplemental Fig. S5).
FIGURE 1.
Overexpression and subcellular distribution of HA-Bcl-xL. A, extracts from untransfected (–) or two clones of HA-Bcl-xL stably transfected cell lines (+) were subjected to immunoblotting with Bcl-xL or HA antibody. GAPDH was used as the loading control. Molecular mass standards are indicated. B, KB-3 and KB-3/HA-Bcl-xL cells were untreated or treated with 30 nm or 200 nm vinblastine (VBL), respectively, for the times indicated. Cytosolic and mitochondrial fractions were prepared and subject to immunoblotting for Bcl-xL. Immunoblotting of procaspase 3 and Cox II (Complex IV) were used as markers for cytosolic and mitochondrial subcellular fractions. C, cytosolic extracts, as in panel B, were probed for expression of active caspase-3.
FIGURE 3.
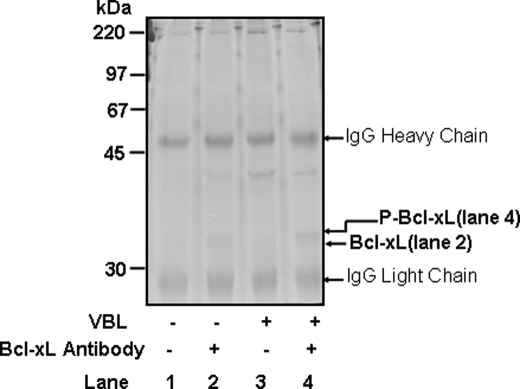
Immunopurification of Bcl-xL. KB-3 cells were untreated (lanes 1 and 2) or treated with 200 nm VBL for 24 h (lanes 3 and 4). Immunoprecipitations using 8 mg of protein were performed with (+Ab, lanes 2 and 4) or without (–Ab, lanes 1 and 3) Bcl-xL antibody. Immunoprecipitates were run on a long (15-cm separation gel) 13.5% acrylamide gel and stained with colloidal Coomassie Blue. The unphosphorylated and phosphorylated HA-Bcl-xL bands are indicated as are IgG light and heavy chains derived from the IgG beads.
FIGURE 2.
HA-Bcl-xL-overexpressing cells are resistant to vinblastine. A, KB-3 or KB-3/HA-Bcl-xL cells were subjected to MTT cell viability assay after treatment with the indicated concentrations of vinblastine for 72 h. The results shown are the mean ± S.D. (n = 6). B, KB-3 or KB-3/HA-Bcl-xL cells were untreated or treated with VBL (30 nm, 48 h) and subject to apoptosis assays as described under “Experimental Procedures.” Values on the y axis represent absorbance at 405 nm. The results shown are mean ± S.D. (n = 6).
To purify phosphorylated Bcl-xL from KB-3/HA-Bcl-xL cells, it was important to establish conditions for optimal phosphorylation. Treatment of cells with 30 nm vinblastine produced a characteristic phosphorylation-induced mobility shift in endogenous Bcl-xL, but any such change in HA-Bcl-xL was obscured by overexpression (supplemental Fig. S2A). However, by reducing gel loading to 3 μg protein and increasing the concentration of vinblastine to 200 nm, a distinct quantitative mobility shift in HA-Bcl-xL was observed (supplemental Fig. S2B). These results together with those of Fig. 1 suggest that the characteristics of overexpressed HA-Bcl-xL parallel those of the endogenous protein, thus validating the system for its intended use.
Purification of HA-Bcl-xL—Initially HA affinity chromatography was tested to purify HA-Bcl-xL, but yields were poor, and many impurities were apparent. Therefore, we investigated immunoprecipitation as a purification approach. Optimization was carried out by testing different amounts of antibody, and under appropriate conditions quantitative recovery of Bcl-xL in the precipitate could be achieved (supplemental Fig. S3). The procedure was scaled up, as described under “Experimental Procedures.” Immunoprecipitated material was released from the beads under high pH conditions, concentrated by rotary evaporation, and subjected to SDS-PAGE and Coomassie staining (Fig. 3). Complete immunoprecipitations performed with Bcl-xL antibody showed stained bands at 32–34 kDa, with the sample from vinblastine-treated cells (lane 4) showing a distinct mobility shift compared with the sample from control cells (lane 2). Prominent bands at 25 and 50 kDa were also observed, but as these were present also in incubations performed in the absence of Bcl-xL antibody (lanes 1 and 3), they presumably represented light and heavy chains derived from the IgG beads.
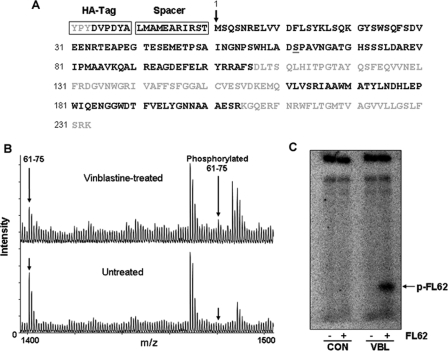
Mass Spectrometry—Excised gel bands were processed and subjected to mass spectrometric analysis as described under “Experimental Procedures.” Overall sequence coverage was 64% (Fig. 4A). To detect Bcl-xL peptides phosphorylated upon vinblastine treatment, a theoretical mass list of all possible phosphorylated versions of Bcl-xL peptides (containing Ser, Thr, or Tyr) was generated. We manually interrogated each of the MALDI-MS spectra for the presence of these phosphorylated peptides. One peptide (residues 61–75) showed single phosphorylation (+80 Da) upon vinblastine treatment (Fig. 4B). MALDI-MS2 analysis of this peptide revealed the phosphopeptide signature loss of 98 Da (20) and phosphorylated or unphosphorylated peptide fragments which unambiguously mapped phosphorylation to Ser-62 and not the other serines or the threonine (supplemental Fig. S4A) (20). MALDI-MS3 analysis of the –98-Da fragment provided further definitive mapping of the site of phosphorylation to Ser-62 (supplemental Fig. S4B). Interestingly, peptide 61–75 showed complete deamidation of Asn-66, consistent with a molecular mass 1 Da greater than expected (theoretical mass, 1399.653; observed mass, 1480.599, representing mono-phosphorylation and mono-deamidation). Asn-66 is one of two sites in Bcl-xL previously determined to be deamidated (22). The other asparagine in Bcl-xL susceptible to deamidation, Asn-52 (22), also showed complete deamidation (data not shown).
FIGURE 4.
Identification of major phosphorylation site. A, HA-Bcl-xL was digested in-gel with trypsin or separately with endoproteinase AspN to produce peptides for MALDI mass spectrometric analysis which identified the peptides in bold representing 64% Bcl-xL sequence coverage. The HA tag and spacer are indicated, and Ser-62 is underlined. B, MALDI mass spectra of Asp-N-digested HA-Bcl-xL highlighting peptide 61–75 from untreated and vinblastine-treated cells showing a single phosphorylation (mass addition of 80 Da) in the vinblastine-treated sample, whereas no detectable phosphorylation was observed in the untreated control. Peptide 61–75 showed complete deamidation of Asn-66 in both samples (mass addition of 1 Da). C, whole cell extracts were prepared from untreated KB-3 cells (control (CON)) or cells treated for 16 h with 30 nm VBL, and aliquots (5 μg) were incubated under phosphorylation conditions with or without FL62, a peptide harboring the vinblastine-induced phosphorylation site, Ser-62, as described under “Experimental Procedures.” Samples were analyzed by high resolution 16.5% acrylamide Tris/Tricine peptide gels (31). A phosphorimage obtained after 48 h exposure is shown with phosphorylated FL62 indicated.
A Synthetic Peptide Containing Ser-62 Is Phosphorylated by a Vinblastine-activated Kinase—To further confirm the ability of Ser-62 in the flexible loop of Bcl-xL to serve as a site of phosphorylation after vinblastine treatment, a synthetic peptide, termed FL62 and representing human Bcl-xL amino acid residues 58–66 was synthesized and utilized as a substrate, as described under “Experimental Procedures.” As shown in Fig. 4C, FL62 was phosphorylated after incubation with an extract from vinblastine treated cells, but no detectable phosphorylation was observed when an extract from untreated cells was used. Similar results were obtained when extracts were prepared from cells treated with paclitaxel, vincristine, or colchicine (data not shown).
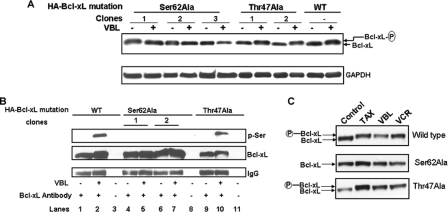
Mutation of Ser-62 Prevents Bcl-xL Phosphorylation—Ser-62 was mutated to alanine, and several clones were generated that overexpressed the mutant protein at a level comparable with wild type. In contrast to wild-type Bcl-xL, Bcl-xL(S62A) failed to undergo a mobility shift in response to vinblastine treatment (Fig. 5A). We also generated cells overexpressing Bcl-xL with Thr-47, another proline-directed site in the loop region of Bcl-xL reported to be phosphorylated by JNK (14), mutated to alanine. The vinblastine-induced mobility shift was preserved in Bcl-xL(T47A), suggesting that the lack of phosphorylation of Bcl-xL(S62A) was due to loss of the phospho-acceptor site and was not a consequence of mutation per se. Furthermore, whereas both wild-type Bcl-xL and Bcl-xL(T47A), after immunoprecipitation after vinblastine treatment, reacted with a phosphoserine antibody, Bcl-xL(S62A) failed to do so (Fig. 5B). This result supported MS data and suggested that Ser-62 is likely the only major site of serine phosphorylation. Other microtubule inhibitors were tested including paclitaxel and vincristine, and these too failed to induce phosphorylation of Bcl-xL(S62A), whereas both wild-type and Bcl-xL(T47A) were phosphorylated normally (Fig. 5C). Thus, Ser-62 is the major site modified in response to microtubule inhibitors in general.
FIGURE 5.
Defective Bcl-xL phosphorylation after mutation specifically of Ser-62. A, KB-3 cell lines expressing equivalent amounts of wild-type (WT) or HA-Bcl-xL(S62A) were untreated or treated with 200 nm vinblastine for 24 h, and extracts were immunoblotted for Bcl-xL with unphosphorylated and phosphorylated (P) forms indicated. Immunoblotting for GAPDH served as a loading control. B, KB-3 cell lines expressing wild-type or HA-Bcl-xL(S62A) were untreated or treated with 200 nm vinblastine for 24 h, and extracts were immunoprecipitated with Bcl-xL antibody and subjected to immunoblotting for phosphoserine (p-Ser) or Bcl-xL as indicated. Note that phosphoserine is only observed after immunoprecipitation of WT Bcl-xL or Bcl-xL(T47A) after vinblastine treatment. Lanes 3, 8 and 11 represent precipitations conducted in the absence of primary antibody. Immunoblotting for IgG was used as an additional control. C, KB-3 cell lines expressing wild-type or HA-Bcl-xL(S62A) were untreated or treated with 200 nm paclitaxel (TAX), VBL, or vincristine (VCR) for 24 h and subjected to immunoblotting for Bcl-xL, with phosphorylated form indicted (P).
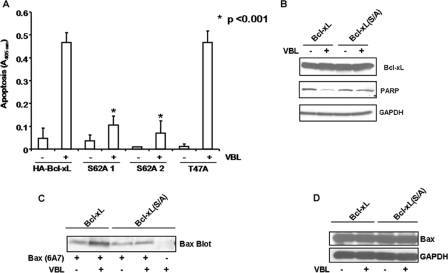
Mutation of Ser-62 Renders Cells Highly Vinblastine-resistant—Assays were conducted to determine whether mutation of Ser-62 affected the apoptotic response to vinblastine. Treatment of KB-3/HA-Bcl-xL cells with 200 nm vinblastine strongly induced apoptosis, whereas two clones expressing Bcl-xL(S62A) were clearly resistant, with less than 20% that of the level of apoptosis under the same conditions (Fig. 6A). Importantly, cells expressing Bcl-xL(T47A) exhibited essentially the same degree of apoptotic sensitivity as cells expressing wild-type Bcl-xL, indicating that the defective apoptotic response of Bcl-xL(S62A) was due to defective phosphorylation and not due to mutation per se. Poly(ADP-ribose) polymerase (PARP) cleavage was evaluated as an additional apoptotic parameter and after vinblastine treatment PARP was less susceptible to cleavage in cells expressing phospho-mutant versus wild-type Bcl-xL (Fig. 6B). In addition, Bax activation was strongly diminished in cells expressing phospho-defective versus wild-type Bcl-xL (Fig. 6C), whereas total Bax expression was unchanged (Fig. 6D). In all cell lines tested, vinblastine treatment promoted cyclin B accumulation, a marker of G2/M phase arrest (supplemental Fig. 5), suggesting that cells expressing Bcl-xL(S62A) undergo mitotic arrest normally. Thus, the diminished apoptotic response is due to a defect in a step subsequent to mitotic arrest.
FIGURE 6.
Ser-62 to Ala mutation of Bcl-xL renders cells highly resistant to vinblastine-induced apoptosis. A, cells expressing wild-type HA-Bcl-xL or two clones expressing the S62A mutant or one clone expressing the T47A mutant were untreated or treated with vinblastine (200 nm, 48 h), and the relative extent of apoptosis was assessed quantitatively using a cell death enzyme-linked immunosorbent assay as described under “Experimental Procedures.” Values on the y axis represent absorbance at 405 nm. The results represent the mean ± S.D. (n = 6) and are representative of two independent experiments. *, significantly different from cells expressing WT Bcl-xL (p < 0.001). B, cells expressing wild-type HA-Bcl-xL or the S62A mutant were untreated or vinblastine-treated (200 nm) for 36 h, and cell extracts were prepared and subjected to immunoblotting for poly(ADP-ribose) polymerase (PARP). The blots were also probed for Bcl-xL and GAPDH expression as indicated. C, cells expressing wild-type HA-Bcl-xL or the S62A mutant were untreated or vinblastine-treated (200 nm) for 36 h, and cell extracts were prepared and subjected to immunoprecipitation with 6A7 active Bax antibody and immunoblotting for Bax. A precipitate prepared in the absence of 6A7 antibody was analyzed as control. D, whole cell extracts were immunoblotted for total Bax expression and reblotted for GAPDH to confirm equal loading.
Bcl-xL Phosphorylation Negatively Modulates Its Interaction with Bax—Previously results have suggested that Bcl-xL can directly interact with Bax and block its pro-apoptotic function (1–4). One attractive possibility is that phosphorylation of Bcl-xL may disrupt its interaction with Bax. To test this hypothesis, cells expressing wild-type Bcl-xL and Bcl-xL(S62A) were untreated or treated with vinblastine, and cell extracts were subjected to Bcl-xL immunoprecipitation followed by immunoblotting for Bcl-xL and Bax. As shown in Fig. 7, the ability of wild-type Bcl-xL to bind Bax was diminished after vinblastine treatment, whereas Bcl-xL(S62A) retained the ability to bind Bax after vinblastine treatment. These results suggest that phosphorylation of Bcl-xL negatively modulates binding to Bax.
FIGURE 7.
Phosphorylation influences Bcl-xL/Bax interaction. Cells expressing wild-type Bcl-xL or Bcl-xL(S62A) were untreated or treated with VBL (200 nm, 36 h), and Bcl-xL was immunoprecipitated. Immunoprecipitations were performed with (lanes 2, 3, 6, and 7) or without (lanes 4 and 8) Bcl-xL antibody. Whole cell extracts were also analyzed (lanes 1 and 5). Samples were analyzed by immunoblotting for Bax (top panel), Bcl-xL (middle panel), and IgG (lower panel).
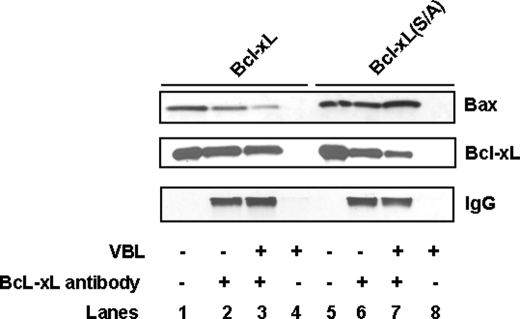
Functional Studies with Phospho-defective and Phosphomimic Bcl-xL—To further probe the function of Bcl-xL phosphorylation, a phospho-mimic Bcl-xL(S62D) mutant was also constructed. KB-3 cells were transiently transfected with a control plasmid or plasmids encoding wild-type, Bcl-xL(S62A), or Bcl-xL(S62D) constructs, and after 48 h, extracts were made and immunoblotted for Bcl-xL. As shown in Fig. 8A, expression levels were comparable. In addition, expression of the proteins was well tolerated, as the relative number of adherent cells was similar under each condition (Fig. 8B). Next, Myc-Bax was co-expressed with each of the three Bcl-xL plasmids. After 48 h, whole cell extracts were made, and immunoblotting for Bax, Bcl-xL, HA-tag, and Myc tag was performed which showed comparable expression levels (Fig. 8C). However, it was evident from visual inspection of the cells that in the case of transfections performed with Bax only or with Bcl-xL(S/D) plus Bax, a majority of the cells were non-adherent and non-viable. Representative phase contrast images highlighting the reduction in adherent cells are shown in Fig. 8D, and quantitation of adherent versus non-adherent cells, representing data from six independent transfections per condition, is shown in Fig. 8E. To determine whether loss of cell viability paralleled effects on Bax binding, co-immunoprecipitations were performed. As shown in Fig. 8F, although wild-type and Bcl-xL(S62A) were competent to bind Bax, a significantly reduced relative amount of Bax co-precipitated with Bcl-xL(S62D). These results suggest that the reduced ability of Bcl-xL(S/D) to counter the adverse effects of Bax expression (Fig. 8D) may be due to a reduced capacity to sequester Bax.
FIGURE 8.
Functional studies of phospho-defective and phospho-mimic Bcl-xL mutants. A, KB-3 cells were transiently transfected with pCDNA3.1 or with HA-Bcl-xL, phospho-defective Bcl-xL(S/A) or phospho-mimic Bcl-xL(S/D) mutant constructs. Extracts were prepared 48 h post-transfection and subjected to immunoblotting for Bcl-xL and GAPDH. B, phase contrast images of KB-3 cells transfected with the respective plasmids at 10× magnification after removal of non-adherent cells. C, KB-3 cells were co-transfected with pCDNA 3.1 or HA-Bcl-xL, phospho-defective Bcl-xL(S/A), or phospho-mimic Bcl-xL(S/D) mutant constructs and Myc-pCMV or Myc-Bax. Transfected cells were harvested 48 h post-transfection and subjected to immunoblotting for Bcl-xL, Bax, HA tag, Myc tag, or GAPDH. D, phase contrast images of adherent cells 48 h after co-transfection with plasmids indicated. E, the adherent and non-adherent cells from each co-transfection were collected separately, counted, and expressed as percentage of the total number of cells. Data represent the averages of six independent determinations with differed by less than 5%. F, immunoprecipitation using Bcl-xL antibody was performed with the lysates from the above co-transfections after 48 h. The immunoprecipitates were subjected to immunoblotting for Bcl-xL or Bax. Immunoblotting for IgG served as a control. E, cell extract.
DISCUSSION
Bcl-xL is a key anti-apoptotic protein that characteristically undergoes phosphorylation in response to microtubule inhibitors (9). Studies designed to elucidate the role of phosphorylation have focused on mutating sites potentially recognized by JNK, which requires a proline residue on the carboxyl side of the phospho-acceptor (23). There are three such sites in Bcl-xL: Ser-62, Thr-47, and Thr-115 (Swiss-Prot entry Q07817). One report suggested Ser-62 to be the site of JNK phosphorylation (16), and another suggested the two threonines to be phosphorylated by JNK (14). However, we have generated compelling evidence that JNK is not responsible for microtubule inhibitor induced Bcl-xL phosphorylation (17). To resolve these uncertainties, we developed a strategy to directly identify vinblastine-induced phosphorylation sites in Bcl-xL. To achieve this goal we took advantage of recent advances in mass spectrometry which enable precise assignment of phosphorylated residues (20, 24). Comparison of MS data of immunopurified Bcl-xL from control and vinblastine-treated cells indicated a major site of phosphorylation at Ser-62, which occurs in the unstructured loop region of the molecule (1–3). Within the sequence covered by the mass spectrometry analysis, there was no evidence of major phosphorylation at other sites. In addition, Bcl-xL(S62A) lacked phosphoserine immunoreactivity after vinblastine treatment (Fig. 5B), and preliminary studies have indicated that phosphorylated Bcl-xL also lacks phosphothreonine immunoreactivity.4 Therefore, it seems unlikely that additional phosphates remained unidentified in the non-covered portion of the sequence, and it appears that Ser-62 is the single major site modified. This contrasts with Bcl-2, which undergoes multisite phosphorylation in response to microtubule inhibitors (10).
Ser-62 is followed by a proline in the Bcl-xL sequence (Fig. 6A), suggesting that the kinase responsible is proline-directed. However, this fact alone is not informative with regard to the identity of the Bcl-xL kinase because over 25% of known Ser/Thr phosphorylation sites are Ser-Pro or Thr-Pro (23), reflecting the widespread occurrence of proline-directed kinases. The best characterized proline-directed protein kinases are mitogen-activated protein kinases and cyclin-dependent protein kinases, and these have been favored as candidates. Data suggesting that JNK is responsible for Bcl-xL phosphorylation are controversial. In DU-145 cells, JNK inhibitor II (SP600125) partially reversed the paclitaxel-induced mobility shift in Bcl-xL, but effects on JNK activity were not reported (16). In KB-3 cells, under conditions validated to strongly inhibit JNK-mediated c-Jun phosphorylation, SP600125 delayed vinblastine-induced Bcl-xL phosphorylation but did not inhibit phosphorylation observed at later time points (17, 25). To resolve these uncertainties, we are currently purifying the kinase using the peptide substrate FL62, shown here to be an effective substrate for a vinblastine-activated kinase (Fig. 4C). Data obtained to date confirm that the kinase is indeed distinct from JNK.5
Cells expressing phospho-defective Bcl-xL were resistant to vinblastine compared with cells expressing comparable levels of wild-type protein. Importantly, cells expressing Bcl-xL carrying a mutation at another potential proline-directed phospho-acceptor site, Thr-47, displayed an essentially identical apoptotic response as cells expressing wild-type Bcl-xL. This is a critical control that eliminates any artifactual effects of mutation and further highlights the key role specifically of Ser-62 in modulating Bcl-xL anti-apoptotic function. Although the findings suggest that phosphorylation normally antagonizes Bcl-xL anti-apoptotic function, they do not address the underlying mechanisms. Previously we showed by co-immunoprecipitation that Bcl-xL and Bax interact in untreated KB-3 cells but not after vinblastine treatment (18). In the present report we have taken advantage of the availability of the phospho-defective mutant to formally demonstrate that phosphorylation negatively modulates Bcl-xL/Bax interaction. Thus, the ability of wild-type Bcl-xL to bind Bax was diminished after vinblastine treatment, whereas phospho-defective Bcl-xL retained the ability to bind Bax after vinblastine treatment. In untreated KB-3 cells, Bax is cytosolic, and Bcl-xL is present both in the cytosol and mitochondrial compartments (17), with Bax translocating to the mitochondria upon vinblastine treatment (18). Thus, it would appear that a key role for cytosolic Bcl-xL in this system is to sequester Bax, and upon Bcl-xL phosphorylation, Bax is freed to translocate to the mitochondria.
Transient transfection of phospho-defective and phosphomimic forms of Bcl-xL provided strong support for the notion that Bcl-xL phosphorylation promotes apoptosis through a mechanism involving release of sequestered Bax. Co-expression of either wild-type or Bcl-xL(S62A) protected cells from Bax-mediated cell demise, but co-expression of Bcl-xL(S62D) failed to provide the same level of protection, in part through a reduced capacity to sequester Bax (Fig. 8). Currently, more comprehensive studies of cells expressing these mutants are under way to further define the role of Bcl-xL phosphorylation in regulation of pro-apoptotic Bcl-2 family members.
MS analysis of peptide 61–75 containing Ser-62 revealed that Asp-66 was deamidated. Another peptide, residues 29–60 derived from AspN digestion, also exhibited complete deamidation of Asn-52. These residues were deamidated in Bcl-xL from both control and vinblastine-treated cells. Deamidation of susceptible asparagines, which are those followed by a glycine in unstructured regions of proteins (26), readily occurs under alkaline conditions (27). Because we used alkaline conditions to elute Bcl-xL from IgG beads, it is entirely possible that deamidation occurred during this step, and the status of these residues in KB-3 cells is, thus, uncertain. Nonetheless, our analysis provides independent biochemical confirmation that Asn-52 and Asn-66 are deamidation-susceptible residues in Bcl-xL. Several studies have indicated that deamidation antagonizes Bcl-xL anti-apoptotic function (22, 28). It is of interest that deamidation occurs in the same loop region as phosphorylation and, like phosphorylation, introduces negative charge. It is tempting to speculate that phosphorylation and deamidation, both of which antagonize Bcl-xL anti-apoptotic function, induce similar structural changes and, thus, functional consequences. Because deamidation occurs in response to DNA damage (22, 28) and phosphorylation in response to microtubule inhibition (9), these distinct modifications may act independently rather than in concert to functionally influence Bcl-xL.
Bcl-xL or Bcl-2 phosphorylation occurs before apoptosis, and it has been postulated that phosphorylation triggers apoptosis. However, more detailed time-course studies using synchronized cells have shown that microtubule inhibitor-induced Bcl-2/Bcl-xL phosphorylation is initiated during mitotic arrest and begins up to 16 h before apoptosis can be first detected (17). Indeed, several reports have shown that apoptosis occurs when Bcl-2/Bcl-xL become dephosphorylated (17, 29, 30). Although these results suggest that phosphorylation does not trigger apoptosis, they by no means exclude the possibility that phosphorylation is a prerequisite for subsequent apoptosis. How can these data be reconciled with results using phospho-defective mutants, which consistently indicate that phosphorylation disables anti-apoptotic activity? One possibility is that apoptosis in response to microtubule inhibitors is minimally a two-step process, with phosphorylation of Bcl-2/Bcl-xL representing a priming mechanism that is required before an additional step(s) can take place. Because phospho-defective molecules cannot complete the first step, subsequent steps will also be impaired. Current studies are focused on defining the relevant molecular events between the onset of phosphorylation and the onset of apoptosis to test the validity of this model.
Supplementary Material
Acknowledgments
We thank Christopher Lyle for excellent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant CA109821 (NCI). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
Footnotes
The abbreviations used are: JNK, c-Jun NH2-terminal kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HA, hemagglutinin; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; MS, mass spectrometry; MALDI, matrix-assisted laser desorption ionization; VBL, vinblastine; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
M. Upreti and T. C. Chambers, unpublished observation.
D. T. Terrano and T. C. Chambers, manuscript in preparation.
References
- 1.Youle, R. J., and Strasser, A. (2008) Nat. Rev. Mol. Cell Biol. 9 47–59 [DOI] [PubMed] [Google Scholar]
- 2.Danial, N. N. (2007) Clin. Cancer Res. 13 7254–7263 [DOI] [PubMed] [Google Scholar]
- 3.Letai, A. G. (2008) Nat. Rev. Cancer 8 121–132 [DOI] [PubMed] [Google Scholar]
- 4.Mollinedo, F., and Gajate, C. (2003) Apoptosis 8 413–450 [DOI] [PubMed] [Google Scholar]
- 5.Rowinsky, E. K., and Donehower, R. C. (1998) The Chemotherapy Source Book, 2nd Ed., pp. 387–423, Williams and Wilkins, Baltimore, MD
- 6.Jordan, M. A., and Wilson, L. (2004) Nat. Rev. Cancer 4 253–265 [DOI] [PubMed] [Google Scholar]
- 7.Bhalla, K. N. (2003) Oncogene 22 9075–9086 [DOI] [PubMed] [Google Scholar]
- 8.Haldar, S., Jena, N., and Croce, C. M. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 4507–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poruchynsky, M. S., Wang, E. E., Rudin, C. M., Blagosklonny, M. V., and Fojo, T. (1998) Cancer Res. 58 3331–3338 [PubMed] [Google Scholar]
- 10.Ruvolo, P. P., Deng, X., and May, W. S. (2001) Leukemia 15, 515–522 [DOI] [PubMed] [Google Scholar]
- 11.Srivastava, R. K., Mi, Q., Hardwick, M. J., and Longo, D. L. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 3775–3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto, K., Ichijo, H., and Korsmeyer, S. J. (1999) Mol. Cell. Biol. 19 8469–8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brichese, L., Barboule, N., Heliez, C., and Valette, A. (2002) Exp. Cell Res. 278 101–111 [DOI] [PubMed] [Google Scholar]
- 14.Kharbanda, S., Saxena, S., Yoshida, K., Pandey, P., Kaneki, M., Wang, Q., Cheng, K., Chen, Y., Campbell, A., Sudha, T., Yuan, Z., Narula, J., Weichselbaum, R., Nalin, C., and Kufe, D. (2000) J. Biol. Chem. 275 322–327 [DOI] [PubMed] [Google Scholar]
- 15.Amundson, S. A., Myers, T. G., Scudiero, D., Kitada, S., Reed, J. C., and Fornace, A. J., Jr. (2000) Cancer Res. 60 6101–6110 [PubMed] [Google Scholar]
- 16.Basu, A., and Haldar, S. (2003) FEBS Lett. 538 41–47 [DOI] [PubMed] [Google Scholar]
- 17.Du, L., Lyle, C. S., and Chambers, T. C. (2005) Oncogene 24 107–117 [DOI] [PubMed] [Google Scholar]
- 18.Upreti, M., Lyle, C. S., Skaug, B., Du, L., and Chambers, T. C. (2006) J. Biol. Chem. 281 15941–15950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, D. H., Sarbassov, D. D., Ali, S. M., King, J. E., Latek, R. R., Erdjument-Bromage, H., Tempst, P., and Sabatini, D. M. (2002) Cell 110 163–175 [DOI] [PubMed] [Google Scholar]
- 20.Chang, E. J., Archambault, V., McLachlin, D. T., Krutchinsky, A. N., and Chait, B. T. (2004) Anal. Chem. 76 4472–4483 [DOI] [PubMed] [Google Scholar]
- 21.Tackett, A. J., DeGrasse, J. A., Sekedat, M. D., Oeffinger, M., Pout, M. P., and Chait, B. T. (2005) J. Proteome Res. 4 1752–1756 [DOI] [PubMed] [Google Scholar]
- 22.Deverman, B. E., Cook, B. L., Manson, S. R., Niederhoff, R. A., Langer, E. M., Rosová, I., Kulans, L. A., Xiaoyun, F., Weinberg, J. S., Heinecke, J. W., Roth, K. A., and Weintraub, S. J. (2002) Cell 111 51–62 [DOI] [PubMed] [Google Scholar]
- 23.Ubersax, J. A., and Ferrell, J. E., Jr. (2007) Nat. Rev. Mol. Cell Biol. 8 530–541 [DOI] [PubMed] [Google Scholar]
- 24.Han, J., Pope, M., Borchers, C., and Graves, L. M. (2002) Anal. Biochem. 310 215–218 [DOI] [PubMed] [Google Scholar]
- 25.Du, L., Lyle, C. S., Obey, T. B., Gaarde, W. A., Muir, J. A., Bennett, B. L., and Chambers, T. C. (2004) J. Biol. Chem. 279 11957–11966 [DOI] [PubMed] [Google Scholar]
- 26.Robinson, N. E., and Robinson, A. B. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 12409–12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson, B. A., Shirokawa, J. M., and Aswad, D. A. (1989) Arch. Biochem. Biophys. 268 276–286 [DOI] [PubMed] [Google Scholar]
- 28.Zhao, R., Oxley, D., Smith, T. S., Follows, G. A., Green, A. R., and Alexander, D. R. (2007) PLOS Biol. 5 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling, Y., Tornos, C., and Perez-Soler, R. (1998) J. Biol. Chem. 273 18984–18991 [DOI] [PubMed] [Google Scholar]
- 30.Ibrado, A. M., Liu, L., and Bhalla, K. (1997) Cancer Res. 57 1109–1115 [PubMed] [Google Scholar]
- 31.Schagger, H. (2006) Nat. Protoc. 1 16–22 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.