Abstract
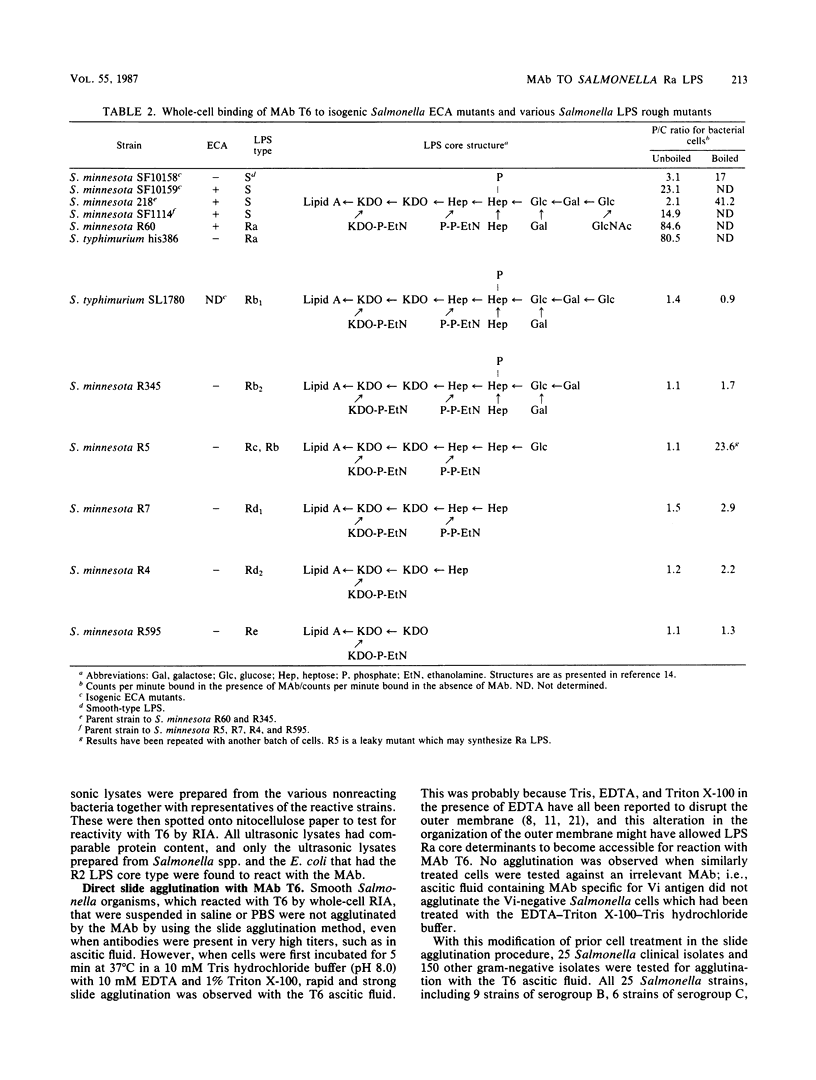
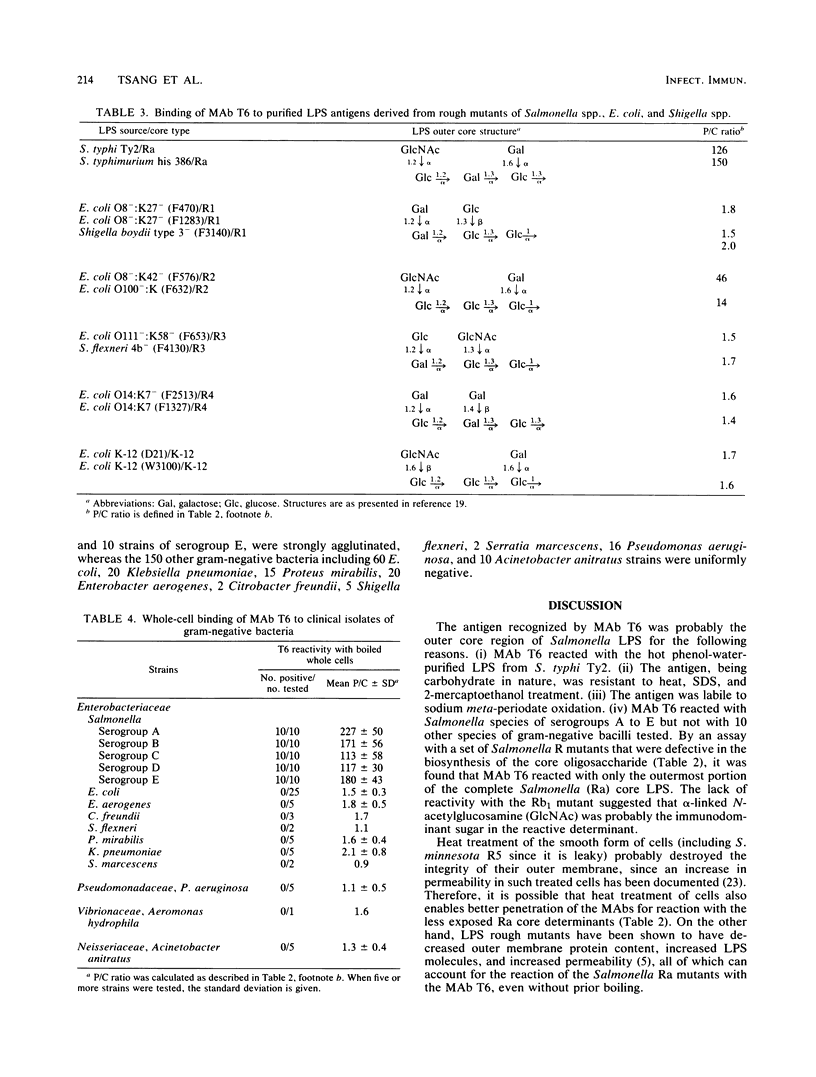
Immunoglobulin G3 murine monoclonal antibody T6 specific for the lipopolysaccharide of Salmonella O serogroups A to E was established. By using R mutants of Salmonella spp., Escherichia coli, and Shigella spp., the major reactive epitope with T6 was tentatively identified as the terminal disaccharide, N-acetylglucosamine 1.2----alpha glucose, of the core oligosaccharide. T6 was reactive with 10 clinical isolates of each of the Salmonella O serogroups A to E but not with 58 isolates of other gram-negative bacteria. Its selective reactivity against Salmonella spp. renders T6 a potentially more useful reagent than the conventional polyvalent serum for the identification of Salmonella spp. It may also serve as a useful molecular tool for the study of the outer core structure of all Salmonella and related species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gibbs P. A., Patterson J. T., Murray J. G. The fluorescent antibody technique for the detection of Salmonella in routine use. J Appl Bacteriol. 1972 Sep;35(3):405–413. doi: 10.1111/j.1365-2672.1972.tb03716.x. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Shenep J. L. Failure of monoclonal antibodies to core glycolipid to bind intact smooth strains of Escherichia coli. J Infect Dis. 1985 Jun;151(6):1005–1011. doi: 10.1093/infdis/151.6.1005. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular organization of the outer membrane of Salmonella typhimurium. Eur J Biochem. 1979 Feb 1;93(3):609–620. doi: 10.1111/j.1432-1033.1979.tb12861.x. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Costerton J. W. Tris(hydroxymethyl)aminomethane buffer modification of Escherichia coli outer membrane permeability. J Bacteriol. 1981 Mar;145(3):1397–1403. doi: 10.1128/jb.145.3.1397-1403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Mohr H. K., Trenk H. L., Yeterian M. Comparison of fluorescent-antibody methods and enrichment serology for the detection of Salmonella. Appl Microbiol. 1974 Feb;27(2):324–328. doi: 10.1128/am.27.2.324-328.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles M. J., Niswander C. A. Mouse monoclonal antibodies reactive with J5 lipopolysaccharide exhibit extensive serological cross-reactivity with a variety of gram-negative bacteria. Infect Immun. 1984 Dec;46(3):677–681. doi: 10.1128/iai.46.3.677-681.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn W. R., Lesmana M., Edwards E. A. Enrichment culture coagglutination test for rapid, low-cost diagnosis of salmonellosis. J Clin Microbiol. 1980 Aug;12(2):151–155. doi: 10.1128/jcm.12.2.151-155.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht S., Fromme I. Züchtung von Salmonella-R-Mutanten in Submerskultur 1. Einfluss der Belüftungsintensität auf die Ausbeuten an Zellmasse und Lipopolysaccharide, die chemische Zusammensetzung und die serologischen Eigenschaften der Lipopolysaccharide. Zentralbl Bakteriol Orig A. 1977 Oct;239(2):178–188. [PubMed] [Google Scholar]
- Schmidt G., Fromme I., Mayer H. Immunochemical studies on core lipopolysaccharides of Enterobacteriaceae of different genera. Eur J Biochem. 1970 Jun;14(2):357–366. doi: 10.1111/j.1432-1033.1970.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Sperber W. H., Deibel R. H. Accelerated procedure for Salmonella detection in dried foods and feeds involving only borth cultures and serological reactions. Appl Microbiol. 1969 Apr;17(4):533–539. doi: 10.1128/am.17.4.533-539.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchido T., Katsui N., Takeuchi A., Takano M., Shibasaki I. Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl Environ Microbiol. 1985 Aug;50(2):298–303. doi: 10.1128/aem.50.2.298-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal O., Jann K., Himmelspach K. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog Allergy. 1983;33:9–39. [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- Young L. S., Stevens P., Ingram J. Functional role of antibody against "core" glycolipid of Enterobacteriaceae. J Clin Invest. 1975 Oct;56(4):850–861. doi: 10.1172/JCI108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]


