Abstract
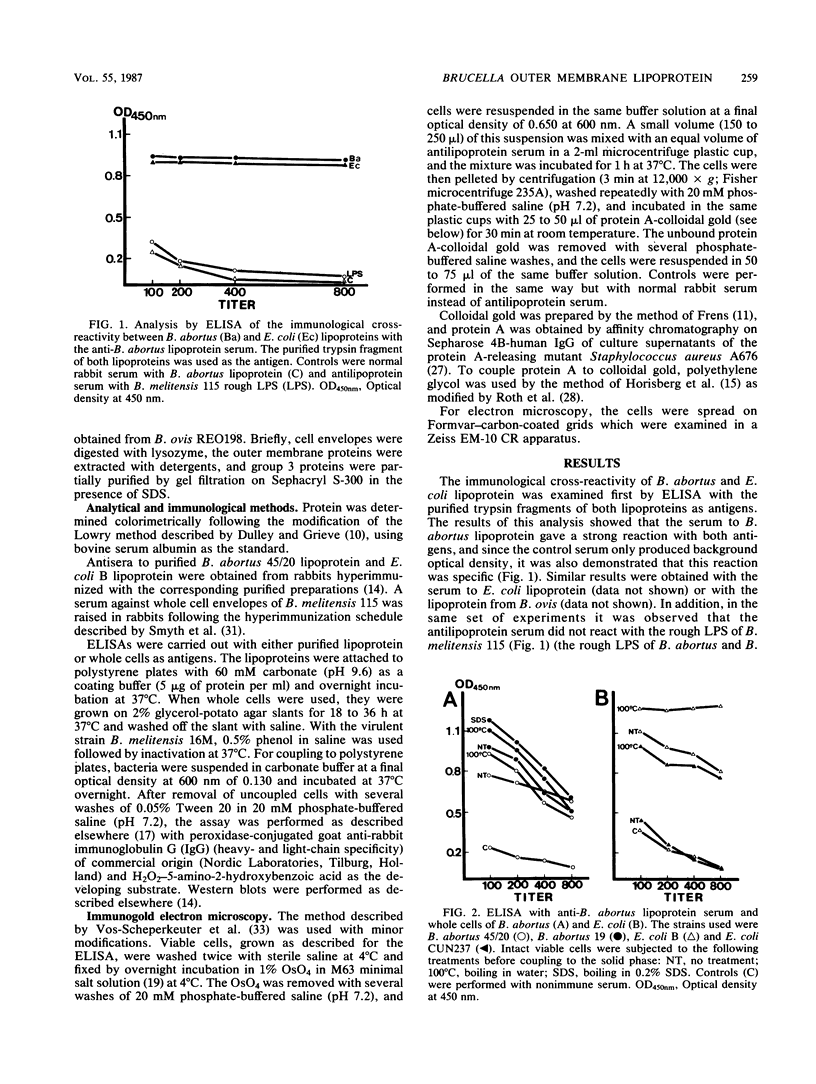
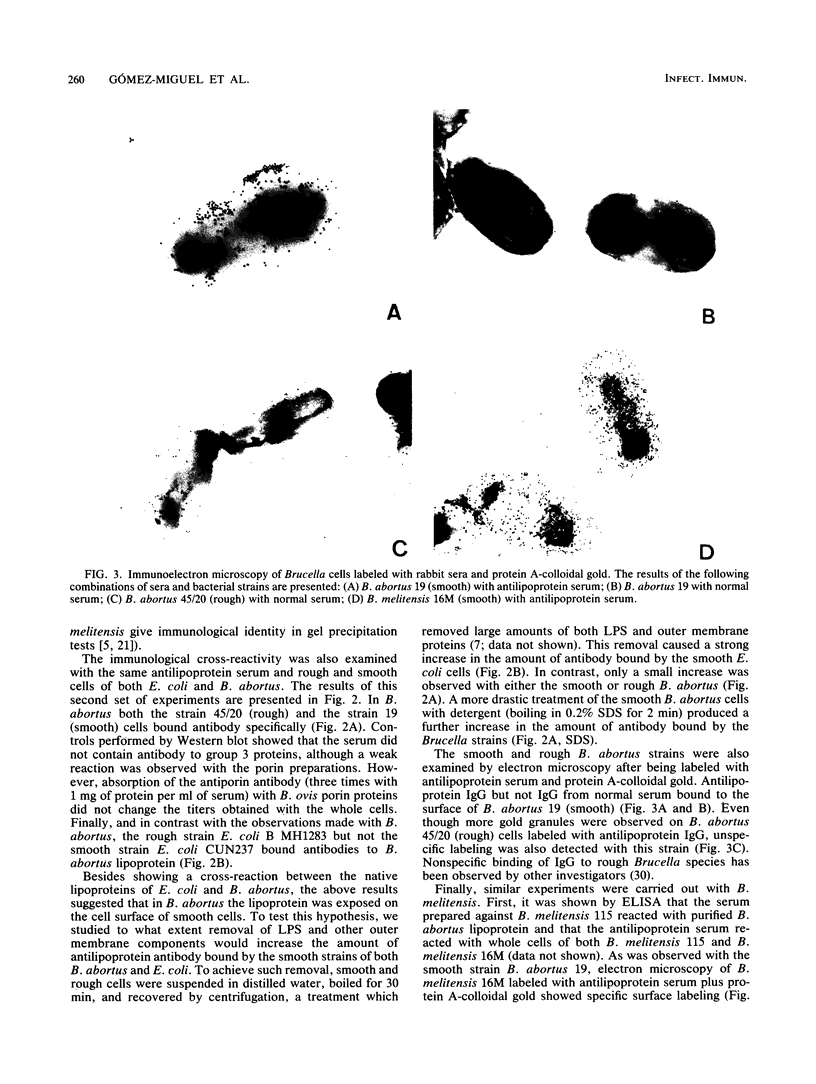
In an enzyme-linked immunosorbent assay (ELISA), purified Brucella abortus and Escherichia coli peptidoglycan-linked lipoproteins gave a strong cross-reaction with sera from rabbits hyperimmunized with the heterologous lipoprotein. When smooth E. coli cells were used as ELISA antigens, the immunological cross-reaction was not observed unless the cells were treated to remove lipopolysaccharide and other outer membrane components. In contrast, intact cells from smooth strains of B. abortus and Brucella melitensis bound anti-lipoprotein immunoglobulin G, and the controls performed by ELISA showed that this reaction was not due to antibodies to the lipopolysaccharide, group 3 outer membrane proteins, or porins. Electron microscopy of cells labeled with antilipoprotein serum and protein A-colloidal gold showed specific labeling of smooth cells from both B. abortus and B. melitensis, even though unspecific labeling by nonimmune serum was observed with rough B. abortus. These results confirm the close similarity between E. coli and Brucella peptidoglycan-linked lipoproteins and show that, in contrast to E. coli, the lipoprotein of B. abortus and B. melitensis is partially exposed on the surface of smooth cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- DeMartini M., Inouye M. Interaction between two major outer membrane proteins of Escherichia coli: the matrix protein and the lipoprotein. J Bacteriol. 1978 Jan;133(1):329–335. doi: 10.1128/jb.133.1.329-335.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Wilson J. B. Antigenic relationship of the gram-negative organism causing canine abortion to smooth and rough brucellae. J Bacteriol. 1968 Feb;95(2):618–624. doi: 10.1128/jb.95.2.618-624.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Toyos J., Salvó M. D., Pardo M. L. A simple method for the extraction of polysaccharide B from Brucella cells for use in the radial immunodiffusion test diagnosis of bovine brucellosis. Ann Rech Vet. 1981;12(1):35–39. [PubMed] [Google Scholar]
- Dubray G., Plommet M. Structure et constituants des Brucella. Caractérisation des fractions et propriétés biologiques. Dev Biol Stand. 1976;31:68–91. [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O., Kim Y. B., Watson D. W. Biological activities of lipid A complexed with bovine-serum albumin. Eur J Biochem. 1972 Dec 4;31(2):230–233. doi: 10.1111/j.1432-1033.1972.tb02524.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Miguel M. J., Moriyón I. Demonstration of a peptidoglycan-linked lipoprotein and characterization of its trypsin fragment in the outer membrane of Brucella spp. Infect Immun. 1986 Sep;53(3):678–684. doi: 10.1128/iai.53.3.678-684.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J., Bauer H. Colloidal gold granules as markers for cell surface receptors in the scanning electron microscope. Experientia. 1975 Oct 15;31(10):1147–1149. doi: 10.1007/BF02326761. [DOI] [PubMed] [Google Scholar]
- Jones L. M., Diaz R., Berman D. T. Endotoxic activity of rough organisms of Brucella species. Infect Immun. 1976 Jun;13(6):1638–1641. doi: 10.1128/iai.13.6.1638-1641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb V. L., Jones L. M., Schurig G. G., Berman D. T. Enzyme-linked immunosorbent assay for bovine immunoglobulin subclass-specific response to Brucella abortus lipopolysaccharides. Infect Immun. 1979 Oct;26(1):240–247. doi: 10.1128/iai.26.1.240-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Moreno E., Jones L. M., Berman D. T. Immunochemical characterization of rough Brucella lipopolysaccharides. Infect Immun. 1984 Mar;43(3):779–782. doi: 10.1128/iai.43.3.779-782.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Effects of nonionic, ionic, and dipolar ionic detergents and EDTA on the Brucella cell envelope. J Bacteriol. 1982 Nov;152(2):822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Isolation, purification, and partial characterization of Brucella abortus matrix protein. Infect Immun. 1983 Jan;39(1):394–402. doi: 10.1128/iai.39.1.394-402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moryión I., Berman D. T. Interaction of Escherichia coli matrix protein with Brucella abortus peptidoglycan and chitin. Dev Biol Stand. 1984;56:227–234. [PubMed] [Google Scholar]
- Nakamura K., Pirtle R. M., Inouye M. Homology of the gene coding for outer membrane lipoprotein within various Gram-negative bacteria. J Bacteriol. 1979 Jan;137(1):595–604. doi: 10.1128/jb.137.1.595-604.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Weir E. M., Leaver C. J., Titus D. E., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 3. IN VITRO TRANSLATION AND CHARACTERIZATION OF FOUR GLYOXYSOMAL ENZYMES. Plant Physiol. 1980 Jan;65(1):40–46. doi: 10.1104/pp.65.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Santos J. M., Verstreate D. R., Perera V. Y., Winter A. J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984 Oct;46(1):188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G., Pringle A. T., Breese S. S., Jr Localization of brucella antigens that elicit a humoral immune response in Brucella abortus-infected cells. Infect Immun. 1981 Dec;34(3):1000–1007. doi: 10.1128/iai.34.3.1000-1007.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Friedman-Kien A. E., Salton M. R. Antigenic analysis of Neisseria gonorrhoeae by crossed immunoelectrophoresis. Infect Immun. 1976 Apr;13(4):1273–1288. doi: 10.1128/iai.13.4.1273-1288.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., Hofnung M., Witholt B. High-sensitivity detection of newly induced LamB protein on the Escherichia coli cell surface. J Bacteriol. 1984 Aug;159(2):435–439. doi: 10.1128/jb.159.2.435-439.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Lipoprotein-bearing peptidoglycan sacculus as a preferred site for the in vitro assembly of membrane from dissociated components of outer membrane of Escherichia coli K-12. J Biochem. 1977 Jun;81(6):1889–1899. doi: 10.1093/oxfordjournals.jbchem.a131651. [DOI] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Reconstitution of an ordered structure from major outer membrane constituents and the lipoprotein-bearing peptidoglycan sacculus of Escherichia coli. J Bacteriol. 1978 Sep;135(3):1024–1031. doi: 10.1128/jb.135.3.1024-1031.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



