Abstract
Group fission is an important dispersal mechanism for philopatric adults. In Cypress Hills Interprovincial Park, Saskatchewan, tree-roosting big brown bats (Eptesicus fuscus) exhibit fission–fusion roosting behaviour. During 2004–2007, the majority of females previously resident to roosting area 1 (RA1) moved to a new roosting area (RA4). We examined how genetic relationships, inferred from data for microsatellite loci and mitochondrial DNA, influenced new roost area (RA) selection during 2006 when colony members were split between the RAs. We found that females who moved to RA4 had higher average relatedness than those that remained in RA1. We found that nearly all females belonging to matrilines with high average relatedness moved to RA4 while females from matrilines with low average relatedness were split between the two RAs. These results suggest that closely related maternal kin preferentially move to new RAs. However, daily roosting preferences within a RA are not based on genetic relationships probably because daily roosting associations between kin and non-kin are used to ensure adequate roost group size. Studying the effects of kinship on the fission and movements of groups not only enhances our understanding of social behaviour and population genetics but also informs conservation decisions.
Keywords: group movement, dispersal, fission–fusion, Eptesicus fuscus, roost area selection, site fidelity
1. Introduction
The genetic structure of social groups is influenced by the behaviour of individuals with respect to mating, dispersal and new group formation (Storz 1999). Dispersal is generally defined as any movement between habitat patches (Bowler & Benton 2005) and is often conceptualized as a young individual moving between patches. However, there are occasions when adult social animals disperse together and form a new group either within their old territory or in a new area (e.g. Greenwood 1980; Pusey & Packer 1987; Clutton-Brock 1989; Isbell et al. 1990; Isbell & Van Vuren 1996). For social groups of philopatric adults and females in matrilineal societies, dispersal usually occurs through group fission.
Group fission has been reported for numerous mammals including naked mole rats (Heterocephalus glaber, Brett 1991), yellow-bellied marmots (Marmota flaviventris, Armitage & Schwartz 2000), Cape ground squirrels (Xerus inauris, Waterman 2002), hyenas (Crocuta crocuta, Holekamp & Smale 1995), African elephants (Loxodonta africana, Archie et al. 2006) and many primates (Van Horn et al. 2007 and references therein). In general, fission occurs when the original group becomes progressively less cohesive until two or more independent groups form (e.g. Widdig et al. 2006). Group fission provides females with the opportunity to change the costs and benefits associated with group living by selecting a new group during a fission event (Van Horn et al. 2007). Although matrilineal kin often remain together during fission events (Packer et al. 1991; Gompper et al. 1998), studies on several species of primates and rodents demonstrate that females do leave matrilineal kin during fission events (Van Horn et al. 2007 and references therein). When M. flaviventris undergoes group fission, females leave matrilineal kin to increase direct fitness through increased reproductive success (Armitage & Schwartz 2000). For savannah baboons (Papio cynocephalus), the composition of the original group influences female choice during a fission event; when groups are composed of numerous matrilineal kin, females remain with matrilineal kin, but when groups are composed of few matrilineal kin, fission occurs across matrilines (Van Horn et al. 2007).
Many mammalian groups are characterized by a fluid social system called fission–fusion sociality. Fission–fusion sociality describes groups that dissolve into smaller units (fission) and merge into larger groups (fusion) with regularity. The frequency of fission and fusion events depends on the costs and benefits of belonging to smaller versus larger groups. When newborn calves are vulnerable to predators, groups of female African elephants (L. africana) fuse to form larger aggregations that are more effective at defending calves (Archie et al. 2006). Individuals within fission–fusion societies frequently make decisions about leaving and joining groups, which provides an opportunity to examine the ecological and social factors that influence decisions about group membership and movements (e.g. Wittemyer et al. 2005; Smith et al. 2007).
During the summer months, several temperate zone tree-roosting bat species exhibit fission–fusion social structure (e.g. Kerth & König 1999; O'Donnell 2000; Willis & Brigham 2004; Russo et al. 2005; Popa-Lisseanu et al. 2007; Rhodes 2007). Members of a maternity colony remain loyal to a particular area of forest, but use many different trees within that area as roosts. Frequent moves between roosts (hereafter roost switching) within these areas are thought to increase knowledge of potential roost trees (Kerth & Reckardt 2003), maintain colony cohesiveness (Willis & Brigham 2004) and/or reduce parasite loads (Reckardt & Kerth 2007) as reviewed by Lewis (1995).
The movement of a colony of tree-roosting bats employing fission–fusion to a new roosting area (RA) has never been documented. If close relatives prefer to move together during fission events, these movements may accelerate genetic differentiation between colonies because kin-structured movements produce groups composed of a non-random selection of individuals from the population (Storz 1999). Here, we take advantage of data from a long-term study of forest-living big brown bats (Eptesicus fuscus) to describe how relatedness and matrilineal relationships influence RA selection during the movement of a maternity colony to a new RA during 2004–2007. During 2006, the previously cohesive colony was separated into two spatially distinct RAs. Our objective was to examine the distribution of kin between the old and new RAs. We asked four specific questions. First, did the movement produce one RA with higher average pairwise relatedness than the other? Second, did females from the same matriline have higher average relatedness than expected at random? Third, how were matrilineal females distributed between the two RAs? Fourth, did belonging to a matriline with high or low average relatedness influence the likelihood of moving to the new RA? Based on general patterns reported about primate behaviour (Van Horn et al. 2007 and references therein), we predicted that bats would move with matrilineal kin and this would result in higher average relatedness in the new RA compared with bats remaining in the original area.
2. Material and methods
(a) Study species
Roosting behaviour by groups of tree-roosting E. fuscus in the Cypress Hills Interprovincial Park, Saskatchewan, Canada has been well documented (Kalcounis & Brigham 1998; Willis et al. 2003, 2006; Willis & Brigham 2004; Metheny et al. 2008). These bats conform to a fission–fusion system of roosting behaviour and exhibit female philopatry (Willis & Brigham 2004). During the summer reproductive season, adult females roost in cavities of trembling aspen trees (Populus tremuloides; Kalcounis & Brigham 1998; Willis et al. 2003, 2006; Willis & Brigham 2004) in social groups consisting of adult breeding females, non-breeding females and young of the year (Willis & Brigham 2004). Females are loyal to the same RA, and many of the same trees, within and between years (Willis et al. 2003; Willis & Brigham 2004). Females and young appear to leave the study site in the autumn for unknown hibernation sites. Three non-overlapping RAs have been previously described (RA1, RA2 and RA3; fig. 1 in Willis & Brigham 2004). RA1 has been the most intensively sampled and consists of approximately 30–45 adult females that move between trees within the RA about every 2 days (Willis & Brigham 2004). Females exhibit preferences for roosting with other individuals (Willis & Brigham 2004), but this preference is not influenced by relatedness or matrilineal relationships (Metheny et al. 2008). The underlying reasons for, and benefits of, roost-mate preferences are not known.
(b) Movement to new RA and monitoring
While studying the roosting behaviour of bats in RA1 during 2004, some bats with radio transmitters could not be located. After intensive searches for these signals, we located them in a new RA (henceforth RA4) approximately 7 km southeast of RA1. Although searching for ‘lost’ transmitter signals had occurred in previous years, bats had never been tracked to this area prior to 2004. In addition, despite intensive trapping of the adult female bats at foraging sites throughout the study area (e.g. Kalcounis & Brigham 1998; Willis & Brigham 2004), captured females were never tracked to RA4.
We continued to monitor bats within RA1 and RA4 during 2004–2007 using the same methods previously used during long-term studies of these bats (Kalcounis & Brigham 1998; Willis et al. 2003, 2006; Willis & Brigham 2004; Metheny et al. 2008). From May to September, bats were trapped using a modified harp trap or mist nets set at roost sites in RA1 and/or RA4 about every two weeks or caught in mist nets set in foraging areas (Willis & Brigham 2004). We radio tagged and radio tracked bats as described by Willis & Brigham (2004) and Metheny et al. (2008). Captured bats were tagged with numbered split ring plastic forearm bands (National Band and Tag Company, Newport, KY) and injected subcutaneously with Trovan ID-100 implantable transponders (Eidap Inc., Sherwood Park, AB). Upon capture, the identity and age of each bat was recorded. Juveniles were distinguished from adults based on the fusion of phalangeal epiphyses (Anthony 1988). For all individuals we took two wing punches (3 mm diameter) and stored them in saturated NaCl solution with 20% DMSO (Vonhof et al. 2006) or ethanol (80–95%). Cavity entrances were monitored with automated reader units (Ediap Inc., Sherwood Park, AB) that detect and store transponder codes with a time and date stamp. We monitored a number of roost trees in RA1 with seven reader units in 2004, continually moving the readers to trees with radio-tagged bats within RA1 in an attempt to always have a reader at roost sites known to be in use. In 2004, when we first found bats using RA4, we moved two reader units from RA1 to RA4. In 2005–2007, instead of moving reader units from tree to tree, we left readers at the same roost trees in RA1 (four in 2005, one in 2006 and zero in 2007) and in RA4 (three in 2005, six in 2006 and seven in 2007) for the entire summer. During 2006–2007, RA4 was intensively sampled with automated reader units at the expense of sampling in RA1. During these 2 years, we were confident that bats detected in RA4 spent the majority of their time roosting in this area but we cannot say definitively whether they occasionally visited RA1 or not. Regardless of whether a bat detected in RA4 occasionally roosted in RA1, bats detected in RA4 still moved to a new RA and the movement to the new RA is the focus of this study. All field methods and animal handling protocols were approved by the University of Regina President's Committee on Animal Care in accordance with the Guidelines of the Canadian Council on Animal Care.
(c) Relatedness and matrilines
We used microsatellite loci to estimate relatedness between individuals and mitochondrial DNA sequences to determine haplotypes of individuals and infer matrilineal relationships (Metheny et al. 2008). Complete details of the genetic markers are described by Metheny et al. (2008). Briefly, DNA was extracted using a DNeasy tissue extraction kit (QIAGEN). Nine microsatellite loci were amplified by polymerase chain reaction (PCR). We used a MegaBACE v. 500 sequencer and Fragment Profiler to size fragments. Pairwise relatedness estimates from the nine microsatellite loci were calculated with Relatedness v. 5.0.8 (Queller & Goodnight 1989) with the same background allele frequencies used by Metheny et al. (2008). For each adult, a portion of the mitochondrial DNA control region (HVII) was PCR amplified using the primers L16517 (Fumagalli et al. 1996) and sH651 (Castella et al. 2001). Sequencing was performed using a MegaBACE v. 500 sequencer and an ET Dye Terminator Cycle Sequencing Kit for MegaBACE DNA Analysis Systems (GE Healthcare). Primers used for PCR amplification amplify a 6 bp repeating region after the first 300 bp (as in Fumagalli et al. 1996; Castella et al. 2001). A reverse primer was designed (5′-ATGCGTATGTCCTGAGACCA-3′) to sequence the first 300 bp before the repeat region. We used L16517 as the forward primer. BioEdit (Hall 1999) was used to align sequences using the ClustalW multiple alignment feature (Thompson et al. 1994). Individual bats with the same sequence belong to the same haplotype, and assuming no mutations or paternal leakage we infer that individuals with the same haplotype belong to the same matriline. Note that we lack the detailed pedigree necessary to define matriline in a similar manner to the definitions often used in primate studies where matriline is defined as clusters of females including a matriarch and her offspring, and matriarch is defined as a living female whose ancestors are all dead (as in Caron-Lormier et al. 2006).
(d) Statistical analysis
(i) Genetic variation
Genetic variation for the microsatellite loci, described as the number of alleles per locus (A), observed heterozygosity (Ho) and expected heterozygosity (He), was calculated using Cervus v. 2.0 (Marshall et al. 1998). For mitochondrial DNA sequences, gene diversity (h), nucleotide diversity (π) and the number of haplotyes (Nh) were calculated using Arlequin v. 2.0 (Schneider et al. 2000).
(ii) Distribution of kin
To answer our first question of whether movement produced one RA with higher average pairwise relatedness than the other, we used a two-group randomization test to compare the average relatedness of individuals using the two RAs during 2006. The two-group randomization test determined whether the difference in average pairwise relatedness between the remnants of the original colony in RA1 and those bats that moved to RA4 was different than expected when randomly assigning individuals to RAs (Manly 1991). For each of 1000 iterations, all bats present in RA1 and RA4 during 2006 were pooled and then randomly assigned to two groups of equal size to the observed number of bats in RA1 and RA4. During 2006, two bats were observed to roost in both RA1 and RA4, and we included these in our totals for both RA1 and RA4 in all analyses. We accounted for these two bats in the randomization test by randomly assigning two bats from the resampled RA1 group to also roost in the resampled RA4 group.
To answer our second question of whether females from the same matriline had higher average relatedness than expected at random, we determined which matrilines had high average relatedness using a randomization test to determine whether individuals with the same haplotype had higher average pairwise relatedness than expected by chance (Manly 1991). We did a randomization test for each haplotype. Each randomization test consisted of 1000 iterations where all the adult females present in RA1 and RA4 during 2006 were pooled and then randomly assigned to a group of equal size to the observed number of bats sharing the given haplotype.
To answer the third question of how matrilineal females were distributed between the two RAs, we examined the distribution of matrilineal females between RA1 and RA4. When multiple females from the same matriline were distributed (i.e. split) between RAs, we used Fisher's exact probability test to evaluate the difference in average relatedness between females in RA1 and RA4 that shared the same matriline. Fisher's exact probability test was not appropriate when zero or one female from a matriline was in one RA because the average relatedness of that RA could not be calculated.
To answer the fourth question of whether belonging to a matriline with high or low average relatedness influenced the likelihood of moving to the new RA, we assessed whether females belonging to matrilines with high average relatedness were more likely to move to RA4 than females belonging to matrilines with low average relatedness. We used a 2×2 contingency table to investigate whether the likelihood of moving to RA4 was influenced by whether a bat was from a haplotype with high average relatedness or low average relatedness.
3. Results
(a) Movement to new RA
Based on movements and recaptures determined from bats carrying radio transmitters, trapping bats and transponder codes, females moved from RA1 to RA4 from 2004 to 2007. Group members from RA1 began the move by starting to use trees in RA4 as day roosts during July 2004. During 2005, many group members were day roosting in both RA1 and RA4, although some females were never found in RA4 (table 1). Only one bat recorded in RA4 was never detected in RA1, otherwise all adult members of RA4 were once resident in RA1 (table 1) or descendants of previous RA1 residents. During 2006, most members were either detected only in RA1 (n=7) or only in RA4 (n=16) with the exception of two bats that were detected in both RA1 and RA4 (table 1). We included these two bats in both RA1 (n=9) and RA4 (n=18) totals. In 2007, three bats found only in RA1 during the previous years roosted in RA4, and two bats found only in RA4 the previous year were radio tracked to RA1 where one was found roosting with the other six bats detected in RA1 during 2007 (table 1). In all years of the study (2004–2007), at least two bats were detected using both RAs.
Table 1.
Haplotype (H), individual identification (band ID) and RAs used by adult females from 2002 to 2007. (‘X’ is used to indicate residency in RA1 before the move (2002–2004), ‘B’ indicates the bat was born in the area that year, ‘+’ indicates the bat was detected in both RA1 and RA4, ‘1’ indicates the bat was detected only in RA1, and ‘4’ indicates the bat was detected in only RA4. Most bats located in both areas in 2005 were only in RA4 during 2006, while bats only in RA1 during 2005 remained in RA1 during 2006. Most bats in RA4 during 2006 stayed for 2007, while bats only in RA1 during 2006 stayed in RA1, went to both areas, or were found only in RA4. Bats born after 2005 and bats present in 2005 but not recorded in 2006 are not shown.)
| band ID | H | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 |
|---|---|---|---|---|---|---|---|
| 91 | H09 | X | X | X | + | 4 | 4 |
| 115 | H09 | X | X | + | 4 | 4 | |
| 84 | H09 | X | X | X | + | 4 | + |
| 158 | H09 | X | X | + | + | 4 | |
| 238 | H09 | B | 4 | ||||
| 256 | H09 | B | 4 | 4 | |||
| 90 | H10 | X | X | X | + | 4 | 4 |
| 181 | H10 | X | X | + | 4 | 4 | |
| 192 | H10 | X | + | 4 | 4 | ||
| 239 | H10 | B | 4 | 4 | |||
| 7 | H12 | X | X | X | 1 | 1 | + |
| 86 | H15 | X | X | X | + | 4 | 4 |
| 155 | H15 | X | X | + | 4 | + | |
| 193 | H15 | X | + | + | + | ||
| 18 | H15 | X | X | X | 1 | 1 | 1 |
| 255 | H15 | B | 4 | 4 | |||
| 157 | H16 | X | X | + | 4 | 4 | |
| 153 | H16 | X | X | 1 | 1 | ||
| 41 | H17 | X | X | X | + | 4 | 4 |
| 82 | H17 | X | X | X | + | 4 | 4 |
| 98 | H17 | X | X | X | + | 4 | 4 |
| 168 | H17 | B | X | + | 1 | 1 | |
| 202 | H17 | B | X | 1 | 1 | + | |
| 138 | H17 | X | X | X | 1 | 1 | 4 |
| 100 | H17 | X | X | X | 1 | 1 | 1 |
(b) Distribution of kin
We found highly polymorphic microsatellite markers and six unique mitochondrial DNA haplotypes (see tables 1, 2 Metheny et al. 2008; table 2). We based the following calculations on adult females present in 2006 when 92% (23 out of 25) of females were found in only one RA.
Table 2.
Maternal genetic diversity described by the number of haplotypes (Hn), the haplotype diversity (h) and the nucleotide diversity (π). Sample size (N) is also listed.
| roost area | N | Hn | h | π |
|---|---|---|---|---|
| RA1 2004 | 48 | 6 | 0.799 | 0.0273 |
| RA1 2006 | 9 | 5 | 0.806 | 0.0351 |
| RA4 2006 | 18 | 5 | 0.817 | 0.0232 |
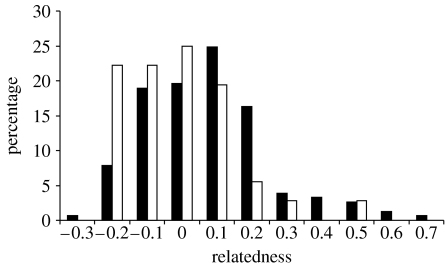
The answer to our first question was that movement produced one RA with higher average pairwise relatedness than the other. The average relatedness of bats roosting in RA4 (r=0.024, n=18) was significantly higher (p=0.008, two-group randomization test) than the average relatedness of bats roosting in RA1 (r=−0.065, n=9, figure 1).
Figure 1.
Percentage of pairs with the binned average relatedness for bats in RA4 (filled bars) compared with RA1 (open bars) during 2006.
The answer to our second question was that females from the same matriline had higher average relatedness than expected at random for three out of five matrilines. Three matrilines (H09, H10 and H16) had higher average pairwise relatedness than expected by chance (randomization test, table 3) and we refer to these haplotypes as having ‘high’ average relatedness. The other two haplotypes (H15 and H17) did not have higher average pairwise relatedness than expected by chance (randomization test, table 3) and we refer to these haplotypes as having ‘low’ average relatedness.
Table 3.
Average pairwise relatedness of adult females within each matriline in RA1 and RA4 during 2006. (Total refers to all the adult females from both RA1 and RA4 during 2006, the asterisks indicate that a bat visited both RA1 and RA4 during 2006 and is included in totals for RA1 and RA4, H indicates haplotype number, n is the number of adult females, r is the average pairwise relatedness, P1 indicates the p-value from the Fisher's exact probability test to detect a difference in average pairwise relatedness between RA4 and RA1, and P2 indicates the p-value from the randomization test to determine whether the average pairwise relatedness of each haplotype was greater than expected by chance. Italics indicates a p-value <0.05.)
| H | RA1 | RA4 | total | |||||
|---|---|---|---|---|---|---|---|---|
| n | r | n | r | P1 | n | r | P2 | |
| H09 | 1 | 6 | 0.133 | 6* | 0.133 | 0.010 | ||
| H10 | 0 | 4 | 0.222 | 4 | 0.222 | 0.003 | ||
| H16 | 1 | 2 | 0.434 | 3 | 0.405 | 0.001 | ||
| H12 | 1 | 0 | 1 | |||||
| H15 | 2 | −0.266 | 3 | 0.101 | 0.083 | 4* | −0.074 | 0.820 |
| H17 | 4 | −0.046 | 3 | 0.462 | 0.029 | 7 | 0.038 | 0.157 |
| total | 9 | −0.065 | 18 | 0.024 | 0.008 | 25 | −0.007 | |
The answer to our third question was that with the exception of two individuals, bats belonging to haplotypes with high average relatedness (H09, H10 and H16) moved as a group to RA4 (table 3). Females belonging to haplotypes with low average relatedness were divided between RA1 and RA4 (H15 and H17; table 3). For individuals belonging to H15 and H17, individuals that remained in RA1 had lower average relatedness than those that moved to RA4 (table 3); however, the difference was not significant for H15 because in the Fisher's exact probability test the observed difference was ranked 1 out of the 12 possible outcomes, given the number of individuals and their genotypes (p-value=1/12=0.083).
The answer to our fourth question was that the likelihood of moving to RA4 was influenced by whether a bat was from a haplotype with high average relatedness (H09, H10 and H16) or low average relatedness (H15 and H17). We found that membership to haplotypes with high or low average relatedness influenced the likelihood of moving to RA4 (2×2 contingency table, Χ2=3.87, p=0.049). Specifically, bats from haplotypes with high average relatedness were more likely to move to RA4 (n=12 moved, n=2 stayed) while bats from haplotypes with low average relatedness were distributed equally between staying in RA1 (n=6) and moving to RA4 (n=6).
4. Discussion
We classified the movement of individuals, over multiple years, from one RA to another and evaluated whether relatedness and matrilineal relationships influenced RA area selection during 2006, when females were split between the two RAs. First, we found that females who moved to RA4 had higher average relatedness than those that remained in RA1. Second, three matrilines had higher average relatedness than expected at random while two did not have higher average relatedness than expected at random. Third, we found that nearly all females belonging to matrilines with high average relatedness moved to RA4 while females from matrilines with low average relatedness were split between the two RAs. Fourth, females belonging to matrilines with high average relatedness were more likely to move to RA4. Closely related matrilineal females moved to RA4 while females with few closely related matrilineal group members stayed in RA1 probably resulting in higher average relatedness among bats in RA4 compared with bats remaining in RA1. Our results suggest that the presence of closely related maternal kin is important for colonization of unfamiliar RAs.
For tree-roosting bats, group movements are likely to be influenced by the number and type of available roost trees within a RA. Tree-roosting bats must have alternative roosting sites available in the event that preferred sites fall down or become home to another animal (e.g. red squirrels Tamiasciurus hudsonicus, J. D. Methany 2004–2005, personal observations). The new RA is considerably closer to the foraging area bats regularly use (Arbuthnott & Brigham 2007) and roost sites in this area might have been used as night roosts prior to their use as day roosts. The pattern of night roosts becoming day roosts has been suggested for the Indiana bat (Myotis sodalis, Kurta et al. 2002) and demonstrated for the Bechstein's bat (M. bechsteinii, Kerth & Reckardt 2003). In many bats, including E. fuscus, mother–daughter pairs share the same night roosts (Brigham & Brigham 1989; Rossiter et al. 2002); therefore, knowledge of night roost sites are probably shared among matrilineal females.
Matrilineal females with shared knowledge of night roosts could facilitate movements of a maternal lineage to a new RA. The presence of a matriarch in elephant and primate societies influences the strength of social bonds within the maternal lineage and facilitates matrilineal cohesiveness during fission events (e.g. Chepko-Sade & Sade 1979; Wittemyer et al. 2005). Social cohesion appears to be greater among closely related compared with distantly related maternal kin. Several primate studies demonstrate the importance of ‘connector’ females and close relatedness for maintaining social cohesion of maternal kin (e.g. Chepko-Sade & Olivier 1979; Chepko-Sade & Sade 1979). Rossiter et al. (2002, 2005) suggested that greater horseshoe bats (Rhinolophus ferrumequinum) engage in mate fidelity and intralineage polygyny to increase levels of relatedness and possibly strengthen social cohesiveness among matrilineal females and increase the inclusive benefits gained through cooperation within matrilines. This might explain why females from matrilines with high average relatedness moved together while those from matrilines with low average relatedness were split between the RAs.
The influence of matrilineal relationships on the movement of individuals to a new RA is surprising given that the adult female E. fuscus do not appear to base daily roosting associations on genetic relationships (Metheny et al. 2008). Within a RA, individuals are usually spatially segregated during the day into smaller groups roosting in different trees. At night, individuals leave the RA to forage, and when they return they make decisions about where and with whom to roost. Metheny et al. (2008) examined the daily roosting decisions of the bats within RA1, which included many of the same females involved in the present study. Metheny et al. (2008) found that genetic relationships are not important for roosting associations within RA1, whereas here we show that the presence of closely related matrilineal females influence group movement to a new RA. Individuals probably have preferred roosting associations with non-kin and kin because thermoregulatory benefits increase with group size (as discussed by Metheny et al. 2008). Although roosting groups are composed of both non-kin and kin, closely related kin within roosting groups might participate in more cooperative interactions than non-kin (as suggested in Kerth et al. 2002; Rossiter et al. 2002, 2005). Therefore, the presence of closely related maternal kin within the roost might be important for cooperative interactions, while daily roosting associations with both kin and non-kin ensure an adequate roost group size.
Bat colonies are rarely ever composed of only one maternal lineage, probably owing to high juvenile mortality rates, low reproductive rates and/or female dispersal (Burland & Wilmer 2001). We found that closely related maternal kin from several different matrilines moved to the new RA several kilometres away from their previous RA. Information transfer among individuals within colonies is probably an important function of sociality in bat species as information transfer even appears to have promoted the occurrence of male social groups (Safi & Kerth 2007). For tree-roosting Bechstein's bat (M. bechsteinii), females share information about roost sites (Kerth & Reckardt 2003), and decisions about where to roost are based on both individual and group knowledge of roost sites (Kerth et al. 2006). In addition, evening bats (Nycticeius humeralis) transfer information about both foraging and roosting sites (Wilkinson 1992). An experimental study by Ruczyński et al. (2007) demonstrated that conspecific echolocation calls improved noctules' (Nyctalus noctula) ability to find tree holes. The echolocation calls of E. fuscus contain information about individual identity, age (juvenile or adult), family affiliation and sex (Masters et al. 1995; Kazial & Masters 2004). Thus, echolocation calls of E. fuscus at new roost sites might be used to promote use of the new site by other colony members. In addition, each year (2004–2007) at least two bats switched between RAs and may have served to maintain the cohesiveness of the original colony and prevent a permanent fission event from occurring. However, despite automated reader units within RA4, we never detected three individuals in RA4. We need to continue to monitor whether these individuals eventually move to RA4.
The influence of relatedness on the movement of individuals suggests that new colonies of bats are formed when small groups of closely related maternal kin move. Kin-structured colony formation might lead to isolation by distance among maternal markers and possibly even nuclear markers on a larger scale (e.g. Burland et al. 1999). If a maternity colony finds new RAs based on the proximity to foraging sites as suggested here and by others (e.g. Kurta et al. 2002), then close proximity of a resident colony, or a source population, to a disturbed site is essential for successful recolonization. This might explain why bat houses are often occupied more quickly when bats are already resident in an area (e.g. White 2004). Understanding how colonies move to new areas and how relatedness and matrilineal relationships influence colony movements is important for understanding the social behaviour of bats especially when considering management-based conservation decisions about roosting habitat.
Group fission and group movement have mainly been studied serendipitously when a population of interest undergoes group fission or group movement. As a result, we know little about how groups of philopatric females become distributed throughout the landscape. Even in well-studied primate populations, the fate of smaller groups formed by fission is often not known because the smaller groups leave the study area. Studying colonization or founder events is important for understanding how new colony formation impacts the genetic structure of populations and for understanding how populations become re-established in disturbed areas. Future studies would benefit from experimental approaches (as in Kerth et al. (2006) and Meunier et al. (2006)) that monitor all individuals in a group coupled with experimental manipulations to investigate the movements of individuals and groups.
Acknowledgments
All field methods and animal handling protocols were approved by the University of Regina President's Committee on Animal Care in accordance with the Guidelines of the Canadian Council on Animal Care.
We thank K. Kolar, C. Willis, M. Ranalli, D. Arbuthnott, D. Wellman, S. Wellman, J. Kilgour, A. Migaj, E. Gillam, B. Graham, R. Philips and K. Lipscomb for their help in the field. We are grateful to the Nuttall family for access to their land and to Kevin Redden and employees of Fort Walsh for logistic help. Funding was provided by the University of North Carolina at Greensboro to M.C.K.R., a Natural Sciences and Engineering Research Council of Canada grant to R.M.B., a North Carolina Academy of Science Award to J.D.M., and a Nature Regina grant, a University of Regina International Graduate Student Scholarship, and an American Society of Mammalogists Grant-in-Aid to K.J.B. We thank O. Rueppell for help with the Visual Basic programming used in randomization tests. B. Fenton and anonymous reviewers improved earlier versions of this manuscript.
References
- Anthony E.L. Age determination in bats. In: Kunz T.H, editor. Ecological and behavioral methods for the study of bats. Smithsonian Institution Press; Washington, DC: 1988. pp. 47–58. [Google Scholar]
- Arbuthnott D, Brigham R.M. The influence of a local temperature inversion on the foraging behaviour of big brown bats, Eptesicus fuscus. Acta Chiropterol. 2007;9:193–201. doi:10.3161/1733-5329(2007)9[193:TIOALT]2.0.CO;2 [Google Scholar]
- Archie E.A, Moss C.J, Alberts S.C. The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc. R. Soc. B. 2006;273:513–522. doi: 10.1098/rspb.2005.3361. doi:10.1098/rspb.2005.3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage K.B, Schwartz O.A. Social enhancement of fitness in yellow-bellied marmots. Proc. Natl Acad. Sci. USA. 2000;97:12 149–12 152. doi: 10.1073/pnas.200196097. doi:10.1073/pnas.200196097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler D.E, Benton T.G. Causes and consequences of animal dispersal strategies: relating individual behaviour to saptial dynamics. Biol. Rev. 2005;80:205–225. doi: 10.1017/s1464793104006645. doi:10.1017/S1464793104006645 [DOI] [PubMed] [Google Scholar]
- Brett R.A. The population structure of naked mole-rat colonies. In: Sherman P.W, Jarvis J.U.M, Alexander R.D, editors. The biology of the naked mole-rat. Princeton University Press; Princeton, NJ: 1991. pp. 97–136. [Google Scholar]
- Brigham R.M, Brigham A.C. Evidence for association between a mother bat and its young during and after foraging. Am. Midl. Nat. 1989;121:205–207. doi:10.2307/2425674 [Google Scholar]
- Burland T.M, Wilmer J.W. Seeing in the dark: molecular approaches to the study of bat populations. Biol. Rev. 2001;76:389–409. doi: 10.1017/s1464793101005747. doi:10.1017/S1464793101005747 [DOI] [PubMed] [Google Scholar]
- Burland T.M, Barratt E.M, Beaumont M.A, Racey P.A. Population genetic structure and gene flow in a gleaning bat, Plecotus auritus. Proc. R. Soc. B. 1999;266:975–980. doi:10.1098/rspb.1999.0732 [Google Scholar]
- Caron-Lormier G, Masson J.P, Ménard N, Pierre J.S. A branching process, its application in biology: influence of demographic parameters on the social structure in mammal groups. J. Theor. Biol. 2006;238:564–574. doi: 10.1016/j.jtbi.2005.06.010. doi:10.1016/j.jtbi.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Castella V, Ruedi M, Excoffier L. Contrasted patterns of mitochondrial and nuclear structure among nursery colonies of the bat Myotis myotis. J. Evol. Biol. 2001;14:708–720. doi:10.1046/j.1420-9101.2001.00331.x [Google Scholar]
- Chepko-Sade B.D, Olivier T.J. Coefficient of genetic relationship and the probability of intragenealogical fission in Macaca mulatta. Behav. Ecol. Sociobiol. 1979;5:263–278. doi:10.1007/BF00293675 [Google Scholar]
- Chepko-Sade B.D, Sade D.S. Patterns of group splitting within matrilineal kinship. Behav. Ecol. Sociobiol. 1979;5:67–86. doi:10.1007/BF00302696 [Google Scholar]
- Clutton-Brock T.H. Female transfer and inbreeding avoidance in social mammals. Nature. 1989;337:70–72. doi: 10.1038/337070a0. doi:10.1038/337070a0 [DOI] [PubMed] [Google Scholar]
- Fumagalli L, Taberlet P, Favre L, Hausser J. Origin and evolution of homologous repeated sequences in the mitochondrial DNA control region of shrews. Mol. Biol. Evol. 1996;13:31–46. doi: 10.1093/oxfordjournals.molbev.a025568. [DOI] [PubMed] [Google Scholar]
- Gompper M.E, Gittleman J.L, Wayne R.K. Dispersal, philopatry, and genetic relatedness in a social carnivore: comparing males and females. Mol. Ecol. 1998;7:157–163. doi: 10.1046/j.1365-294x.1998.00325.x. doi:10.1046/j.1365-294x.1998.00325.x [DOI] [PubMed] [Google Scholar]
- Greenwood P.J. Mating systems, philopatry, and dispersal in birds and mammals. Anim. Behav. 1980;28:1140–1162. doi:10.1016/S0003-3472(80)80103-5 [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Holekamp K.E, Smale L. Rapid change in offspring sex ratios after clan fission in the spotted hyena. Am. Nat. 1995;145:261–278. doi:10.1086/285739 [Google Scholar]
- Isbell L.A, Van Vuren D. Differential costs of locational and social dispersal and their consequences for female group-living primates. Behaviour. 1996;133:1–36. doi:10.1163/156853996X00017 [Google Scholar]
- Isbell L.A, Cheney D.L, Seyfarth R.M. Costs and benefits of home range shifts among vervet monkeys (Cercopithecus aethiops) in Amboseli National Park, Kenya. Behav. Ecol. Sociobiol. 1990;27:351–358. doi:10.1007/BF00164006 [Google Scholar]
- Kalcounis M.C, Brigham R.M. Secondary use of aspen cavities by tree-roosting big brown bats. J. Wildlife Manage. 1998;62:603–611. doi:10.2307/3802336 [Google Scholar]
- Kazial K.A, Masters W.M. Female big brown bats, Eptesicus fuscus, recognize sex from a caller's echolocation signals. Anim. Behav. 2004;67:855–863. doi:10.1016/j.anbehav.2003.04.016 [Google Scholar]
- Kerth G, König B. Fission, fusion and nonrandom associations in female Bechstein's bats (Myotis bechsteinii) Behaviour. 1999;136:1187–1202. doi:10.1163/156853999501711 [Google Scholar]
- Kerth G, Reckardt K. Information transfer about roosts in female Bechstein's bats: an experimental field study. Proc. R. Soc. B. 2003;270:511–515. doi: 10.1098/rspb.2002.2267. doi:10.1098/rspb.2002.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerth G, Safi K, König B. Mean colony relatedness is a poor predictor of colony structure and female philopatry in the communally breeding Bechstein's bat (Myotis bechsteinii) Behav. Ecol. Sociobiol. 2002;52:203–210. doi:10.1007/s00265-002-0499-6 [Google Scholar]
- Kerth G, Ebert C, Schmidtke C. Group decision making in fission–fusion societies: evidence from two-field experiments in Bechstein's bats. Proc. R. Soc. B. 2006;273:2785–2790. doi: 10.1098/rspb.2006.3647. doi:10.1098/rspb.2006.3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurta A, Murray S.W, Miller D.H. Roost selection and movements across the summer landscape. In: Kurta A, Kennedy J, editors. The Indiana bat: biology and management of an endangered species. Bat Conservation International; Austin, TX: 2002. pp. 118–129. [Google Scholar]
- Lewis S.E. Roost fidelity of bats: a review. J. Mammal. 1995;76:481–496. doi:10.2307/1382357 [Google Scholar]
- Manly B. Chapman and Hall; London, UK: 1991. Randomization and Monte Carlo methods in biology. [Google Scholar]
- Marshall T, Slate J, Kruuk L, Pemberton J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. doi:10.1046/j.1365-294x.1998.00374.x [DOI] [PubMed] [Google Scholar]
- Masters W.M, Raver K.A.S, Kazial K.A. Sonar signals of big brown bats, Eptesicus fuscus, contain information about individual identity, age and family affiliation. Anim. Behav. 1995;50:1243–1260. doi:10.1016/0003-3472(95)80041-7 [Google Scholar]
- Metheny J.D, Kalcounis-Rueppell M.C, Willis C.K.R, Kolar K.A, Brigham R.M. Genetic relationships between roost-mates in a fission–fusion society of tree-roosting big brown bats (Eptesicus fuscus) Behav. Ecol. Sociobiol. 2008;62:1043–1051. doi:10.1007/s00265-007-0531-y [Google Scholar]
- Meunier H, Leca J.B, Deneubourg J.L, Petit O. Group movement decisions in capuchin monkeys: the utility of an experimental study and a mathematical model to explore the relationship between individual and collective behaviours. Behaviour. 2006;143:1511–1527. doi:10.1163/156853906779366982 [Google Scholar]
- O'Donnell C.F.J. Cryptic local populations in a temperate rainforest bat Chalinolobus tuberculatus in New Zealand. Anim. Conserv. 2000;3:287–297. doi:10.1111/j.1469-1795.2000.tb00114.x [Google Scholar]
- Packer C, Gilbert D.A, Pusey A.E, O'Brien S.J. A molecular genetic analysis of kinship and cooperation in African lions. Nature. 1991;351:562–565. doi:10.1038/351562a0 [Google Scholar]
- Popa-Lisseanu A.G, Bontadina F, Mora O, Ibáñez C. Highly structured fission-fusion societies in an aerial-hawking, carnivorous bat. Anim. Behav. 2007;75:471–482. doi:10.1016/j.anbehav.2007.05.011 [Google Scholar]
- Pusey A.S, Packer C. Dispersal and philopatry. In: Smuts B.B, Cheney D.L, Seyfarth R.M, Wrangham R.W, Struhsaker T.T, editors. Primate societies. University of Chicago; Chicago, IL: 1987. pp. 250–266. [Google Scholar]
- Queller D.C, Goodnight K.F. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. doi:10.2307/2409206 [DOI] [PubMed] [Google Scholar]
- Reckardt K, Kerth G. Roost selection and roost switching of female Bechstein's bats (Myotis bechsteinii) as a strategy of parasite avoidance. Oecologia. 2007;154:581–588. doi: 10.1007/s00442-007-0843-7. doi:10.1007/s00442-007-0843-7 [DOI] [PubMed] [Google Scholar]
- Rhodes M. Roost fidelity and fission-fusion dynamics of white-striped free-tailed bats (Tadarida australis) J. Mammal. 2007;88:1252–1260. doi:10.1644/06-MAMM-A-374R1.1 [Google Scholar]
- Rossiter S.J, Jones G, Ransome R.D, Barratt E.M. Relatedness structure and kin-biased foraging in the greater horseshoe bat (Rhinolophus ferrumequinum) Behav. Ecol. Sociobiol. 2002;51:510–518. doi:10.1007/s00265-002-0467-1 [Google Scholar]
- Rossiter S.J, Ransome R.D, Faulkes C.G, Le Comber S.C, Jones G. Mate fidelity and intra-lineage polygyny in greater horseshoe bats. Nature. 2005;437:408–410. doi: 10.1038/nature03965. doi:10.1038/nature03965 [DOI] [PubMed] [Google Scholar]
- Ruczyński I, Kalko E.K.V, Siemers B.M. The sensory basis of roost finding in a forest bat, Nyctalus noctula. J. Exp. Biol. 2007;210:3607–3615. doi: 10.1242/jeb.009837. doi:10.1242/jeb.009837 [DOI] [PubMed] [Google Scholar]
- Russo D, Cistrone L, Jones G. Spatial and temporal patterns of roost use by tree-dwelling barbastelle bats Barbastella barbastellus. Ecography. 2005;28:769–776. doi:10.1111/j.2005.0906-7590.04343.x [Google Scholar]
- Safi K, Kerth G. Comparative analyses suggest that information transfer promoted sociality in male bats in the temperate zone. Am. Nat. 2007;170:465–472. doi: 10.1086/520116. doi:10.1086/520116 [DOI] [PubMed] [Google Scholar]
- Schneider S, Roessli D, Excoffier L. Genetics and Biometry Laboratory, University of Geneva; Geneva, Switzerland: 2000. Arlequin v. 2.000: a software for population genetics data analysis. [Google Scholar]
- Smith J.E, Memenis S.K, Holekamp K.E. Rank-related partner choice in the fission-fusion society of the spotted hyena (Crocuta crocuta) Behav Ecol Sociobiol. 2007;61:753–765. doi:10.1007/s00265-006-0305-y [Google Scholar]
- Storz J.F. Genetic consequences of mammalian social structure. J. Mammal. 1999;80:553–569. doi:10.2307/1383301 [Google Scholar]
- Thompson J.D, Higgins D.G, Gibson T.J. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. doi:10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn R.C, Buchan J.C, Altmann J, Alberts S.C. Divided destinies: group choice by female savannah baboons during social group fission. Behav. Ecol. Sociobiol. 2007;61:1823–1837. doi:10.1007/s00265-007-0415-1 [Google Scholar]
- Vonhof M.J, Barber D, Fenton M.B, Strobeck C. A tale of two siblings: multiple paternity in big brown bats (Eptesicus fuscus) demonstrated using microsatellite markers. Mol. Ecol. 2006;15:241–247. doi: 10.1111/j.1365-294X.2005.02801.x. doi:10.1111/j.1365-294X.2005.02801.x [DOI] [PubMed] [Google Scholar]
- Waterman J.M. Delayed maturity, group fission and the limits of group size in female Cape ground squirrels (Sciuridae: Xerus inauris) J. Zool. Lond. 2002;256:113–120. [Google Scholar]
- White E.P. Factors affecting bat house occupancy in Colorado. Southw. Nat. 2004;49:344–349. doi:10.1894/0038-4909(2004)049<0344:FABHOI>2.0.CO;2 [Google Scholar]
- Widdig A, Nürnberg P, Bercovitch F.B, Trefilov A, Berard J.B, Kessler M.J, Schmidtke J, Streich W.J, Krawczak M. Consequences of group fission for the patterns of relatedness among rhesus macaques. Mol. Ecol. 2006;15:3825–3832. doi: 10.1111/j.1365-294X.2006.03039.x. doi:10.1111/j.1365-294X.2006.03039.x [DOI] [PubMed] [Google Scholar]
- Wilkinson G.S. Information transfer at evening bat colonies. Anim. Behav. 1992;44:501–518. doi:10.1016/0003-3472(92)90059-I [Google Scholar]
- Willis C.K.R, Brigham R.M. Roost switching, roost sharing and social cohesion: forest-dwelling big brown bats, Eptesicus fuscus, conform to the fission-fusion model. Anim. Behav. 2004;68:495–505. doi:10.1016/j.anbehav.2003.08.028 [Google Scholar]
- Willis C.K.R, Kolar K.A, Karst A.L, Kalcounis-Rueppell M.C, Brigham R.M. Medium- and long-term reuse of trembling aspen cavities as roosts by big brown bats (Eptesicus fuscus) Acta Chiropterol. 2003;5:85–90. [Google Scholar]
- Willis C.K.R, Voss C.M, Brigham R.M. Roost selection by forest-living female big brown bats (Eptesicus fuscus) J. Mammal. 2006;87:345–350. doi:10.1644/05-MAMM-A-118R1.1 [Google Scholar]
- Wittemyer G, Douglas-Hamilton I, Getz W.M. The socioecology of elephants: analysis of the processes creating multitiered social structures. Anim. Behav. 2005;69:1357–1371. doi:10.1016/j.anbehav.2004.08.018 [Google Scholar]