Abstract
The epithelium of the adult prostate contains 3 distinct cell types: basal, luminal, and neuroendocrine. Tissue-regenerative activity has been identified predominantly from the basal cells, isolated by expression of CD49f and stem cell antigen-1 (Sca-1). An important question for the field is whether all basal cells have stem cell characteristics. Prostate-specific microarray databases were interrogated to find candidate surface antigens that could subfractionate the basal cell population. Tumor-associated calcium signal transducer 2 (TACSTD2/Trop2/M1S1/GA733-1) was identified because it was enriched after castration, in prostate sphere cells and in the basal fraction. In the murine prostate, Trop2 shows progenitor characteristics such as localization to the region of the gland proximal to the urethra and enrichment for sphere-forming and colony-forming cells. Trop2 subfractionates the basal cells into 2 populations, both of which express characteristic basal cell markers by quantitative PCR. However, only the basal cells expressing high levels of Trop2 were able to efficiently form spheres in vitro. In the human prostate, where Sca-1 is not expressed, sphere-forming progenitor cells were also isolated based on high expression of Trop2 and CD49f. Trop2-expressing murine basal cells could regenerate prostatic tubules in vivo, whereas the remaining basal cells had minimal activity. Evidence was found for basal, luminal, and neuroendocrine cells in prostatic tubules regenerated from Trop2hi basal cells. In summary, functionally distinct populations of cells exist within the prostate basal compartment and an epithelial progenitor can give rise to neuroendocrine cells in vivo.
Keywords: neuroendocrine, progenitor, sphere assay
Many adult tissues of the body contain a rare population of somatic stem cells that can self-renew and differentiate into the mature cell types of the organ (1). Recent studies demonstrating that somatic stem cells can serve as the target for transforming mutations and act as cancer-initiating cells (2) have highlighted the importance of identifying tissue-specific stem cells. The isolation of somatic stem cells and subsequent investigation of their unique properties will be useful for understanding and targeting cancer.
The prostate epithelium is made up of basal, luminal, and neuroendocrine cells. Prostate stem cells are thought to reside within the basal layer because basal cells preferentially survive androgen ablation (3) and basal cells can give rise to luminal cells in vitro (4). The transcription factor p63 is expressed in prostate basal cells, and p63-null animals fail to develop a prostate (5). These data suggest that basal cells may represent or include prostate stem cells and that p63 may be essential for prostate development. However, the p63-null urogenital sinus epithelium forms prostatic tissue with luminal and neuroendocrine cells, but no basal cells (6). Both studies show that p63 plays an important developmental role in the prostate, but demonstrate conflicting data on the role of basal cells during differentiation. Based on expression of integrin α6 (CD49f), Lawson et al. (7) found that the majority of cells in the gland with in vitro and in vivo stem-like activity possessed basal cell characteristics. A fundamental question in the field is whether all basal cells have stem cell characteristics and can give rise to the mature cells of the organ or if only a subset of basal cells have tissue regenerative activity.
The neuroendocrine cell is the rarest epithelial cell type in the adult prostate. In the normal gland, neuroendocrine cells are dispersed within the basal layer (8) and extend processes between adjacent basal and luminal cells (9). Although their role in development and cancer is unclear, neuroendocrine cells are known to secrete neuropeptides that may contribute to hormone-refractory prostate cancer and metastasis through a paracrine mechanism (9–11). Neuroendocrine differentiation occurs in >30% of human prostate cancers (9) and in some mouse models of prostate cancer (12). However, studies correlating neuroendocrine differentiation and tumor grade have given conflicting results (9).
Evidence is lacking to definitively show whether neuroendocrine cells have an ectodermal or endodermal origin (13). Because of their location in the basal layer of prostatic tubules, neuroendocrine cells were believed to originate from an epithelial stem cell (endoderm). Human prostate epithelial progenitors can give rise to neuroendocrine-like cells in vitro (14, 15), and in response to a stimulus such as IL-6, LNCaP cells can adopt a neuroendocrine morphology and express high levels of neuronal markers (16).
An opposing theory is that neuroendocrine cells may have originated from the neural crest and migrated into the prostate epithelium. This theory is supported by the appearance of chromogranin A-positive cells in the embryonic site where the prostate forms, before gland formation, as demonstrated by Aumuller et al. (17). Cells expressing chromogranin A are first seen in the paraganglia flanking the mesenchyme and later in the urogenital mesenchyme. As the gland forms, chromogranin A-positive cells appear in the basal layer of the epithelium (17). However, the demonstration of neuroendocrine cells before prostatic gland formation does not exclude an epithelial origin for neuroendocrine cells found within the gland. In fact, neural crest derived cells may support the development of epithelial-derived neuroendocrine cells (9).
Leong et al. (18) recently demonstrated that enriched murine prostate stem cells could regenerate tissue grafts containing cells that express the neuroendocrine cell marker synaptophysin. The presence of synaptophysin+ cells in grafts under the kidney capsule does not rule out neural crest-derived neuroendocrine cells migrating into prostatic tubules. Lineage tracing experiments are necessary to definitely determine whether epithelial progenitors can give rise to neuroendocrine cells in vivo.
The majority of markers used to isolate progenitors from the prostate are not conserved between mouse and human. In the human prostate, stem/progenitor cells have been enriched based on expression of integrins α2/β1 (19), CD44 (20), or CD133 (21). Prostate stem/progenitor cells have been isolated from the mouse based on expression of stem cell antigen-1 (Sca-1) (22, 23), which is not expressed in the human prostate, and integrin α6/CD49f (7, 24). In a recent report (18), murine prostate stem cells were also isolated by expression of CD44, CD133, and CD117. Leong et al. (18) note that CD117 is expressed in human prostate basal cells and may be a useful marker for isolating human prostate stem/progenitors. The identification of highly-enriched stem/progenitor populations in the mouse prostate is important for investigating the self-renewal properties of stem cells and their role in cancer initiation and propagation. However, the identification of stem/progenitor fractions from the human prostate has greater therapeutic implications. The cancer stem cell hypothesis suggests that a rare population of cancer cells may be responsible for sustaining tumorigenesis (1, 25–27) and these cancer stem cells often share antigenic profiles with normal tissue stem/progenitor cells (28, 29). These studies highlight the importance of identifying primitive cell populations from normal and malignant human prostate tissue, as new treatments may be designed to target cancer stem cells rather than the bulk tumor. Therefore, it would be useful to identify antigens found on stem/progenitor cells from both the mouse and human prostate.
We report the identification of a marker, tumor-associated calcium signal transducer 2 (TACSTD2/Trop2/M1S1/GA733-1) (30, 31), that functionally discriminates between 2 basal cell subpopulations. We demonstrate that not all basal cells share stem cell characteristics, and that basal cells expressing high levels of Trop2 are enriched for in vitro and in vivo stem-like activity. We follow the progeny of labeled cells to provide in vivo evidence that basal, luminal, and neuroendocrine cells are generated from an epithelial progenitor with tri-lineage differentiation potential. Finally, we show that sphere-forming progenitor cells can be isolated from the human prostate by using markers identified in the mouse (Trop2 and CD49f), suggesting a conservation of selected progenitor markers between mouse and human.
Results
Microarray Analysis Identifies Trop2 as a Candidate Progenitor Marker with Stem-Like Characteristics.
We interrogated prostate-specific microarrays (from the literature and at the University of California, Los Angeles) where progenitor genes should be enriched to identify candidate antigens that may subfractionate prostate basal cells. We identified Trop2 as a leading candidate because it was 20-fold enriched in the murine prostate after castration (32), 12-fold enriched in prostate sphere cells compared with the total epithelium (Li Xin, Rita U. Lukacs, and O.N.W., unpublished work), and 2-fold enriched in the basal fraction compared with the remaining epithelium (Rita U. Lukacs, D.A.L., and O.N.W., unpublished work). Trop2 is a type I transmembrane protein in the EpCAM family with no known function or ligand (30, 31). Trop2 expression has been reported in normal and/or malignant tissue from kidney, lung, ovary, testis (33), intestine (34), pancreas (35), and breast (36); however, it has not been previously associated with stem or progenitor cells.
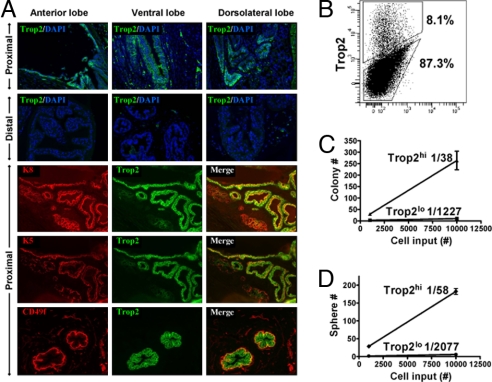
The putative prostate stem cell niche is found in the region closest to the urethra, referred to as the proximal region. Tsujimura et al. (37) used BrdU pulse–chase experiments to demonstrate the majority of label retention in the proximal region of the gland and later showed that cells isolated from this region have higher proliferative capacity in vitro. To determine the expression pattern of Trop2 in the murine prostate, we used both FACS analysis and immunohistochemistry. FACS analysis demonstrates that Trop2 is highly expressed by ≈7–10% of the total prostate cells (Fig. 1B). Immunofluorescent staining on tissue sections revealed that Trop2 is expressed on cytokeratin 5+ (K5) basal cells and cytokeratin 8+ (K8) luminal cells in the proximal region, whereas almost no Trop2+ cells are found in the distal regions (Fig. 1A). In contrast, Trop2 is expressed on the majority of K5+ basal cells in the distal regions after androgen ablation (Fig. S1).
Fig. 1.
Trop2 has progenitor characteristics in the murine prostate. (A) Frozen prostate tissue sections from 8- to 12-week-old mice were stained with antibodies against Trop2 in combination with CD49f, cytokeratin 5, or cytokeratin 8. Sections were counterstained with DAPI (blue) nuclear stain. (B) FACS analysis for Trop2 on total dissociated prostate cells. (C) Trop2hi and Trop2lo prostate cells were isolated by FACS from 8- to 12-week-old mice. Cells were plated on top of a thin layer of Matrigel. Graph shows the colonies formed after 10 days versus the number of cells plated (1,000, 10,000). (D) Trop2hi and Trop2lo prostate cells were isolated by FACS from 8- to 12-week-old mice. Graph shows the spheres formed in Matrigel after 10 days versus the number of cells plated (1,000, 10,000).
To investigate Trop2 as a functional progenitor marker, we turned to 2 in vitro assays that measure primitive cell activity: the prostate colony and sphere assays. Lawson and coworkers (7, 38) demonstrated that both colonies and spheres are clonal in origin, and the basal fraction contains the majority of colony-forming and sphere-forming cells. We isolated Trop2hi and Trop2lo cells by FACS and plated equal numbers of cells into the colony and sphere assays at 2 dilutions. The majority of colony-forming and sphere-forming activity was in the Trop2hi fraction. Linear regression analysis demonstrates that Trop2hi cells can form colonies at a rate of 1/38 and spheres at a rate of 1/58 (Fig. 1 C and D). Expressed on both basal and luminal cells, Trop2 identifies a progenitor population in the murine prostate with proximal localization and enrichment for in vitro activity in 2 independent assays indicative of primitive cell populations.
Trop2 Fractionates Lin−Sca-1+CD49fhi Cells into Two Basal Subpopulations.
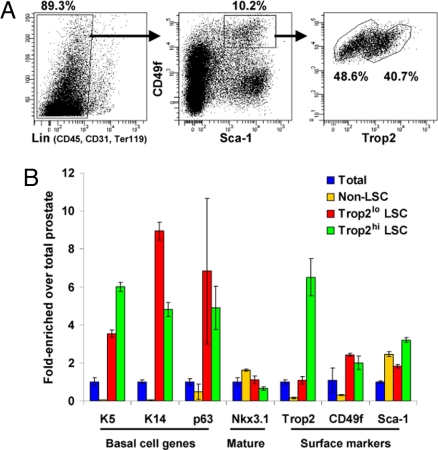
We have previously demonstrated that the stem/progenitor fraction defined by the antigenic profile CD45−CD31−Ter119−Sca-1+CD49fhi (LSC) contains the majority of basal cells from the murine prostate (7). FACS analysis for Trop2 expression within the LSC fraction shows that Trop2 separates the basal cells into 2 relatively equal subpopulations, and that ≈40% of LSC cells express high levels of Trop2 (Fig. 2A). We isolated RNA from these 2 populations (Trop2hi LSC and Trop2lo LSC) and the fraction depleted of LSC cells (non-LSC) and compared their gene expression with the total prostate. Quantitative RT-PCR (qRT-PCR) demonstrates that Trop2 is 6-fold enriched in the Trop2hi LSC cells compared with the Trop2lo fraction. Both populations express the basal markers p63, cytokeratins 5 and 14, and integrin α6 at high levels compared with the total prostate and the non-LSC cells (Fig. 2B). Nkx3.1 is not enriched in the LSC subpopulations, showing that the 2 fractions are depleted of mature markers. Based on these data, the Trop2hi and Trop2lo LSC cells have basal characteristics.
Fig. 2.
Trop2 separates the Lin−Sca-1+CD49fhi fraction into 2 basal subpopulations. (A) FACS plots show gates drawn for sorting of Trop2hi LSC (Lin−Sca-1+CD49fhi) and Trop2lo LSC subpopulations from 8- to 12-week-old mice. (B) RNA was isolated from total cells, non-LSC (Lin− not Sca-1+CD49fhi), Trop2hi LSC, and Trop2lo LSC fractions in duplicate experiments. RNA was synthesized into cDNA and subjected to qRT-PCR. Graph shows fold-enrichment over the total prostate cells for each gene. GAPDH was used as the reference gene.
Trop2 and CD49f Enrich for Progenitors from the Mouse and Human Prostate.
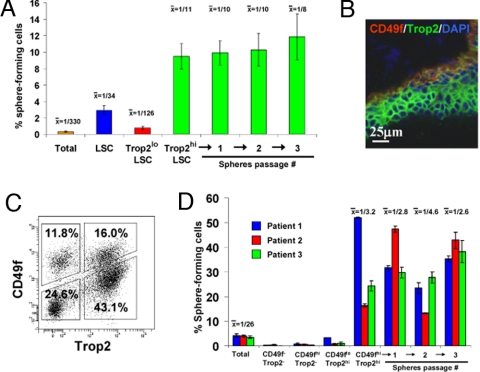
Having confirmed that Trop2 separates the basal fraction into 2 subpopulations that both express basal markers, we asked whether our basal subpopulations are functionally distinct. We isolated the LSC basal population and the further fractionated Trop2hi and Trop2lo basal subpopulations and plated equal cell numbers into the sphere assay. The LSC basal fraction formed spheres at a rate of 1/34, similar to what has been reported (7). The Trop2hi basal fraction further enriched for sphere-forming cells to a frequency of 1/11, a 30-fold enrichment over the total unfractionated cells (1/330), whereas the Trop2lo basal fraction only formed spheres at a rate of 1/132 (Fig. 3A). The residual sphere-forming activity in the Trop2lo basal fraction may be explained by a small number of contaminating cells from the highly active Trop2hi basal fraction. Alternatively, the Trop2lo fraction may contain stem-like cells at a far lower frequency than the Trop2hi fraction. Spheres from the Trop2hi basal fraction could be passaged for >3 generations at a consistent rate of sphere formation to demonstrate self-renewal activity (primary: 1/11; passage 1: 1/10; passage 2: 1/10; passage 3: 1/8). Not only have we further refined the sphere-forming progenitor cell by using Trop2, but we have demonstrated that the majority of stem-like activity is contained within a subpopulation of basal cells.
Fig. 3.
Trop2 enriches for sphere-forming cells from the mouse and human prostate. (A) Total prostate, LSC, Trop2hi LSC, and Trop2lo LSC cells were isolated by FACS from 8- to 12-week-old mice. Graph shows the percentage of sphere-forming cells, based on the spheres formed in Matrigel from each population per 2,500 cells plated (5,000 cells plated from total prostate) after 8 days of growth. Spheres from the Trop2hi LSC subpopulation were dissociated and replated for 3 successive generations. Data from several experiments were pooled. (B) Frozen human prostate tissue sections were stained with antibodies against Trop2 and CD49f. Sections were counterstained with DAPI (blue) nuclear stain. (C) FACS plots show gates drawn for sorting of human prostate cells into 4 subpopulations based on expression of Trop2 and CD49f. (D) Total prostate, CD49f−Trop2−, CD49fhiTrop2−, CD49floTrop2hi, and CD49fhiTrop2hi subpopulations were isolated by FACS from 3 patient samples. Graph shows the percentage of sphere-forming cells, based on the spheres formed in Matrigel from each population per 2,500 cells plated after 7 days of growth. Spheres from the CD49fhiTrop2hi subpopulation were dissociated and passaged for 3 successive generations.
Similar to the prostate sphere assay described in the mouse system (38), primary human prostate cells can form spheres in Matrigel that demonstrate progenitor characteristics, such as self-renewal in vitro and differentiation to the basal and luminal cell types in vivo. Human prostaspheres are clonally derived and predominantly express basal markers. When a mixture of malignant cells with a TMPRSS2-ERG translocation and normal cells (without the translocation) are plated into the assay, the resulting spheres do not retain the translocation, suggesting that the assay selects for normal progenitors (I.P.G., C. Tran, S. Perner, W.S., B. Zhang, L. Xin, C. Head, R. Reiter, M. Rubin, and O.N.W., unpublished work).
Having demonstrated that we can enrich for murine prostate sphere-forming cells by using Sca-1, CD49f, and Trop2, we looked to identify the human prostate sphere-forming cell. Although Sca-1 is not expressed in the human prostate, we found expression of both Trop2 and CD49f. In tissue sections, the highest expression of Trop2 and CD49f is observed along the basal layer of the epithelium (Fig. 3B). FACS analysis shows that Trop2 and CD49f separate the human prostate into 4 populations (Fig. 3C). We collected prostate tissue from multiple patients who underwent prostate removal for cancer. Human prostate tissue specimens were mechanically and enzymatically dissociated into single cells and cultured overnight in serum-free media. Dissociated cells were sorted into 4 populations based on CD49f and Trop2, and equal cell numbers were plated into the sphere assay. Data for 3 representative patients are shown, and the results have been repeated for all patients that have been analyzed (data not shown). The overnight culture enriched for progenitor cells before antigen separation, as the total population sent through the sorter formed spheres at an average rate of 1/26. Upon fractionating the cells, the majority of sphere-forming activity came from the cells expressing high levels of CD49f and Trop2, as seen in the mouse. Cells from the CD49fhiTrop2hi population comprised ≈16% of the total prostate cells after short-term culturing, and this fraction formed spheres at an average rate of 1/3.2, an 8-fold enrichment over the total unfractionated cells (Fig. 3D). Spheres from this enriched fraction could be passaged for up to 3 generations at a sphere-forming rate similar to the original CD49fhiTrop2hi fraction (primary: 1/3.2; passage 1: 1/2.8; passage 2: 1/4.6; passage 3: 1/2.6). Our data show that we can reproducibly isolate sphere-forming progenitor cells from the human prostate by using markers that were identified in the murine prostate, suggesting a conservation of progenitor markers that may be found in other epithelial tissues.
Trop2hi Basal Cells Give Rise to Basal, Luminal, and Neuroendocrine Cells in Vivo.
Previous experiments showed that the Lin−Sca-1+CD49fhi basal fraction could produce prostatic tubules when combined with urogenital sinus mesenchyme (UGSM) and implanted s.c. into immunodeficient mice with Matrigel (7). Regenerated tubules contained basal and luminal cells, but neuroendocrine cells were not evaluated. Recently, neuroendocrine cells were found in tissue regenerated from Lin−Sca-1+ CD133+CD44+CD117+ cells (18); however, experiments were not performed to demonstrate a definitive epithelial origin. To follow the progeny of implanted stem cells, we used transgenic mice expressing either GFP or DsRed under the β-actin promoter. Fluorescently-labeled DsRed+ Trop2hi and Trop2lo basal cells were isolated from β-actin-DsRed mice and combined with 105 total unfractionated GFP+ prostate cells from β-actin–GFP mice. Purified DsRed+ and total GFP+ cells were combined with 2 × 105 UGSM cells and implanted s.c. into SCID mice. After 8 weeks of growth, we harvested grafts and looked for fluorescently-labeled prostatic tubules. We found that whereas all grafts contained an abundance of GFP+ tubules (Fig. 4A2), the Trop2hi basal fraction gave rise to the majority of DsRed+ prostatic tubules (Fig. 4A3). Previous experiments performed without total unfractionated cells yielded similar results at a lower frequency of tubule formation (data not shown).
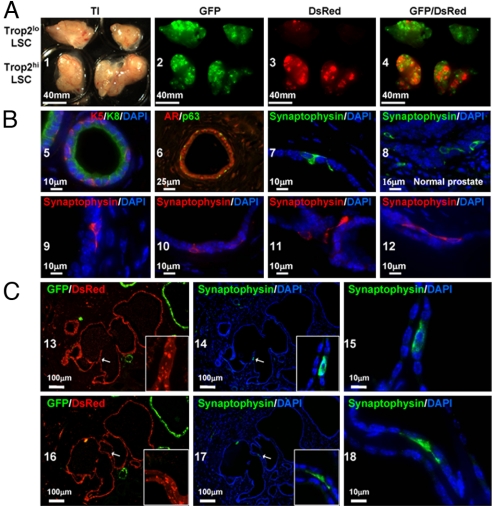
Fig. 4.
Trop2hi LSC cells give rise to basal, luminal, and neuroendocrine cells in vivo. (A) A total of 3 × 103 Trop2hi LSC or Trop2lo LSC cells from β-actin DsRed mice, 105 total prostate cells from β-actin GFP mice, and 2 × 105 UGSM cells were combined in Matrigel and injected s.c. into SCID mice. Grafts were harvested after 8 weeks, and overview images were taken by using transillumination (TI) and DsRed or GFP fluorescence. (B) Tissue sections from regenerated tissue were stained for antibodies against cytokeratin 5 (K5), cytokeratin 8 (K8), androgen receptor (AR), p63, and synaptophysin. Sections were counterstained with DAPI (blue) nuclear stain. Sections from 8- to 12-week-old mice were stained for comparison of neuroendocrine cell morphology and marker expression pattern (synaptophysin). (C) In tissue sections from regenerated tissue, DsRed and GFP fluorescence (13 and 16) were visualized before antigen retrieval caused by a loss of signal after treatment. The remaining images demonstrate synaptophysin staining and DAPI nuclear counterstain after antigen retrieval.
Analysis of sectioned tubules revealed K5+ and p63+ basal cells and K8+ luminal cells. Androgen receptor was detected primarily in luminal cells (Fig. 4 B 5 and 6). In addition to the basal and luminal cells that were previously detected in regenerated tissue, we found rare cells within the basal layer of DsRed+ tubules that expressed the neuroendocrine marker synaptophysin (Fig. 4B7). The membrane localization and extended morphology of regenerated synaptophysin+ cells appeared similar to those found in synaptophysin+ neuroendocrine cells from the adult murine prostate (Fig. 4B8). Within the basal layer of regenerated tubules, we found numerous examples of synaptophysin+ cells extending dendrite-like processes around neighboring epithelial cells (Fig. 4B 9–12), characteristic of neuroendocrine morphology. To quantify the frequency of neuroendocrine cells, we detected an average of 6 synaptophysin+ cells per 66 regenerated tubules in a 4-μm thick tissue section (18 sections counted). In normal prostate tissue sections, many of the synaptophysin+ cells also expressed chromogranin A. We were unable to demonstrate chromogranin A reactivity in regenerated tissue sections, suggesting a lack of full neuroendocrine differentiation. Factors from the native prostatic niche that support neuroendocrine development may be absent in the assay microenvironment. Alternatively, full neuroendocrine differentiation may take longer than the 8-week regeneration process.
Because evidence suggests that prostate neuroendocrine cells have an ectodermal/neural crest origin (17), we looked for synaptophysin+ cells within DsRed+ tubules to demonstrate an epithelial origin for neuroendocrine cells. Only cells derived from the Trop2hi LSC stem/progenitor fraction should express DsRed fluorescence. If neuroendocrine cells migrate into the regenerated tubules from an alternate location, they should not express DsRed. We identified neuroendocrine cells within the basal layer of regenerated tubules with characteristic neuroendocrine morphology (Fig. 4B 15 and 18) that colocalize with DsRed fluorescence (Fig. 4B 13, 14, 16, and 17). By following the progeny of DsRed+ Trop2hi basal cells into DsRed+ synaptophysin+ neuroendocrine cells, we have provided direct evidence that a prostate stem cell can generate 3 epithelial cell types.
Discussion
Stem cells isolated from different epithelial tissues share antigenic profiles, such as integrins α6 and β1, the stem cell antigen Sca-1, and CD24 (7, 39, 40). Similarly, somatic and cancer stem cells have been isolated from numerous human tissues by using the markers CD44 and CD133 (19, 21, 26, 27). We have found 2 markers, Trop2 and CD49f, which are conserved between mouse and human. Because of the similarity of stem cell profiles, the identification of markers such as Trop2 should be useful for multiple tissue systems.
In the mouse prostate, we have shown that a subpopulation of basal cells, expressing high levels of Trop2, have stem cell characteristics. It is unlikely that the spheres and tubules generated by Trop2hi basal cells are different from those generated in previously published studies. We have simply enriched for the cells capable of generating these structures. Although we have now identified neuroendocrine cells and traced their lineage from epithelial progenitors, the purification of stem-like cells is not necessary to regenerate tissue containing neuroendocrine cells. Using different immunohistochemical techniques and antibodies, we can now detect neuroendocrine cells in grafts generated from unfractionated cells (data not shown).
Although Trop2hi basal cells can efficiently form spheres and tubules, the remaining basal cells have minimal activity in vitro and in vivo. Both of these populations express high levels of CD49f and other basal cell genes, but they differ in their functional output. This finding has interesting implications for the epithelial lineage hierarchy in the prostate. Linear models of prostate differentiation have suggested that stem cells in the basal layer give rise to transit-amplifying cells that differentiate into mature luminal cells (14, 41). In our assays, all basal cells do not have stem cell activity. Our data support an alternative branching model, where a subset of basal cells can differentiate into basal, luminal, and neuroendocrine cells. A similar model has been reported in the mammary system, where stem cells give rise to both basal/myoepithelial and luminal progenitors, which differentiate into mature basal and luminal cells. Future identification of a luminal progenitor, as demonstrated in the mammary system (42), would further support the branching model.
Trop2 is highly expressed by a subset of both basal (30–40% Trop2hi) and luminal (5–15% Trop2hi) cells in the murine prostate (Fig. 2A and data not shown). Trop2hi cells are concentrated in the proximal region, the putative prostate stem cell niche. We observed a similar region-restricted expression pattern in murine endometrial glands, where the brightest Trop2 staining was observed in regions of invagination (Fig. S2). The restricted pattern suggests that a localized niche signal, such as the Wnt pathway, may control Trop2 expression. Segditsas et al. (43) showed that Trop2 has high confidence TCF4 binding sites and may be a Wnt target gene in the intestinal epithelium. Trop2 was significantly enriched in early intestinal cancers driven by APC mutations as compared with the normal tissue. A similar pattern was seen with the crypt stem cell marker Lgr5 (Gpr49) and other Wnt target genes (43).
We also found that Trop2 expression is significantly increased during tumorigenesis caused be aberrant PI3K signaling. Thirty-three percent of cells from Pten-null murine prostate tumors were Trop2hi by FACS analysis compared with ≈8% in aged-matched wild-type prostate tissue (Fig. S3). A conserved mechanism in multiple organ systems could regulate Trop2 expression in the normal and malignant niche. Although the function of Trop2 in normal and cancer cells has yet to be elucidated, Trop2 appears to play a role in the tumorigenicity and invasion of cancer cells. Targeting Trop2 in colon cancer cells through RNA interference reduces both soft-agar colony formation and tumor initiation in mice, whereas inhibitory antibodies can block invasion in Matrigel in vitro (44). Trop2 may also have oncogenic activity through a chimeric CYCLIN D1-TROP2 mRNA present in many human cancers (45). The cancer stem cell marker EpCAM (26), a Trop2 family member, is a therapeutic target in a variety of epithelial cancers and likely contributes to oncogenic signaling beyond cell–cell adhesion (46). Trop2 and EpCAM may have similar functions in solid tumors.
Our demonstration that only a subpopulation of basal cells has stem cell characteristics raises some interesting questions about the cell of origin for prostate cancer. Can both basal subpopulations give rise to prostate cancer, or are the more primitive cells the preferred target for transforming mutations that drive tumorigenesis? Can different genetic and epigenetic changes lead to cancer initiation from different basal subpopulations? The finding that prostate stem cells can give rise to basal, luminal, and neuroendocrine cells, and the fact that the majority of human prostate tumors have a predominant luminal phenotype (47) and focal neuroendocrine differentiation (48) supports the theory that prostate cancer can initiate from a progenitor with luminal and neuroendocrine differentiation potential.
Recent reports on the role of stem cells in the initiation and propagation of cancer suggest that it may be important to target prostate cancer at the level of the stem cell (2, 28, 29). Defining and profiling these 2 basal subpopulations should elucidate the mechanisms by which the more primitive basal cells maintain their self-renewal and differentiation potential. The identification of critical self-renewal mechanisms in Trop2hi basal cells may provide new targets for the treatment of prostate cancer.
Materials and Methods
Animals and Tissue Collection.
The wild-type C57BL/6, β-actin GFP (C57BL/6-Tg[ACTbEGFP]1Osb), β-actin DsRed (C57BL/6-Tg[ACTB-DsRed.MST]1Nagy/J), and CB17Scid/Scid mouse strains were purchased from The Jackson Laboratory. Mice were housed and bred under the regulation of the Division of Laboratory Animal Medicine at the University of California, Los Angeles. Prostate cell dissociation was adapted from previously described protocols (49). Prostate tissue was collected from 8- to 12-week-old mice, minced into small fragments, digested with collagenase (GIBCO) as described (49), and digested with 0.05% trypsin/EDTA (Invitrogen) for 5 min at 37 °C. The cell suspension was passed through 18- to 22-gauge syringes several times and filtered through a 40-μm cell strain. UGSM was harvested as described (49).
Immunofluorescent and Histological Analysis.
Immunohistochemical analysis of frozen and paraffin-embedded tissue sections were performed as described (7, 49). Antibodies are listed in SI Text. Sections were counterstained with DAPI (Vector) and analyzed by fluorescent microscopy.
FACS.
Dissociated prostate cells were suspended in DMEM/10% FBS and stained with antibody for 30 min at 4 °C. Antibodies are listed in SI Text. FACS analysis was performed by using BD FACS Canto (BD Biosciences). Cell sorting was done by using BD FACS Vantage and the BD FACS Aria II.
In Vitro Prostate Colony- and Sphere-Forming Assays.
The colony assay was adapted from previously described protocols (7). Isolated epithelial cells were plated on top of a thin layer of Matrigel (BD Biosciences), rather than on irradiated feeder cells. The sphere assay was performed as described (7, 38).
In Vivo Prostate Regeneration.
Dissociated or FACS-isolated prostate cells were counted by hemocytometer and mixed with 2 × 105 UGSM cells as described (7). Cell mixtures were pelleted, resuspended in 40 μL of Matrigel (kept on ice), and injected s.c. on the backs of 8-to 16-week-old CB17Scid/Scid mice by using an insulin syringe. Eight weeks later, grafts were harvested, sectioned, and stained.
RNA Isolation and qRT-PCR.
Sorted cells were collected, spun down, and resuspended in buffer RLT from the RNeasy Micro Kit (Qiagen). The standard RNA isolation protocol was followed. Reverse transcription was performed with a SuperScript III first-strand synthesis system (Invitrogen). qRT-PCR was performed by using iQ SYBR Green Supermix for Real-Time PCR (Bio-Rad) on a Bio-Rad iCycler and iQ5 2.0 Standard Edition Optical System Software. Data were analyzed by using the Pfaffl method. Primer sequences are listed in Table S1.
Human Prostate Tissue Acquisition and Dissociation.
Human prostate tissue was obtained from 3 patients undergoing retropubic prostatectomy for adenocarcinoma of the prostate or cystoprostatectomy for bladder cancer. All subjects provided consent for tissue collection in accordance with an approved protocol through the office for the protection of research subjects at the University of California, Los Angeles. Tissue specimens were placed on ice and brought immediately to the laboratory for mechanical and enzymatic digestion. Prostate tissue was sharply minced into small fragments (1 mm3) in RPMI-1640 medium supplemented with 10% FBS. Tissue fragments were washed once and incubated for 12 h in 0.25% type I collagenase (5 mL/g). Organoids were washed in RPMI-1640 medium and treated with TripLE (Invitrogen) for 5 min at 37 °C. Dissociated tissue cellular suspensions were sequentially filtered through 100- and 40-μm filters. After filtration, cell suspensions were passed through a 23-gauge needle. Cells were plated overnight in PrEGM (Clonetics).
Supplementary Material
Acknowledgments.
We thank Rita Lukacs and Akanksha Chhabra for critical reading of the manuscript; Rita Lukacs, and Li Xin for generation of microarray datasets; Camille Soroudi and Jason Lee for data analysis; Hong Wu and Jing Jiao (University of California, Los Angeles) for Pten-null prostate tissue; and Sanaz Memarzadeh (University of California, Los Angeles) for frozen tissue sections of murine endometrial glands. A.S.G. is supported by Ruth L. Kirschstein National Research Service Award GM07185. O.N.W. is an Investigator of the Howard Hughes Medical Institute and is supported by the Prostate Cancer Foundation and the University of California, Los Angeles, Specialized Program of Research Excellence in Prostate Cancer (Jean deKernion, principal investigator). I.P.G is supported by the Prostate Cancer Foundation and Department of Defense Grant W81XWH-07-2–0030.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811411106/DCSupplemental.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Passegue E, Wagner EF, Weissman IL. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell. 2004;119:431–443. doi: 10.1016/j.cell.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 3.English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11:229–242. doi: 10.1002/pros.2990110304. [DOI] [PubMed] [Google Scholar]
- 4.Robinson EJ, Neal DE, Collins AT. Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate. 1998;37:149–160. doi: 10.1002/(sici)1097-0045(19981101)37:3<149::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Signoretti S, et al. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131:4955–4964. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- 7.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 1999;39:135–148. doi: 10.1002/(sici)1097-0045(19990501)39:2<135::aid-pros9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Chuang CK, Wu TL, Tsao KC, Liao SK. Elevated serum chromogranin A precedes prostate-specific antigen elevation and predicts failure of androgen deprivation therapy in patients with advanced prostate cancer. J Formos Med Assoc. 2003;102:480–485. [PubMed] [Google Scholar]
- 11.Uchida K, et al. Murine androgen-independent neuroendocrine carcinoma promotes metastasis of human prostate cancer cell line LNCaP. Prostate. 2006;66:536–545. doi: 10.1002/pros.20369. [DOI] [PubMed] [Google Scholar]
- 12.Chiaverotti T, et al. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008;172:236–246. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matusik RJ, et al. Prostate epithelial cell fate. Differentiation. 2008;76:682–698. doi: 10.1111/j.1432-0436.2008.00276.x. [DOI] [PubMed] [Google Scholar]
- 14.Litvinov IV, et al. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res. 2006;66:8598–8607. doi: 10.1158/0008-5472.CAN-06-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rumpold H, et al. Neuroendocrine differentiation of human prostatic primary epithelial cells in vitro. Prostate. 2002;53:101–108. doi: 10.1002/pros.10129. [DOI] [PubMed] [Google Scholar]
- 16.Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin-6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aumuller G, et al. Neurogenic origin of human prostate endocrine cells. Urology. 1999;53:1041–1048. doi: 10.1016/s0090-4295(98)00631-1. [DOI] [PubMed] [Google Scholar]
- 18.Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008 doi: 10.1038/nature07427. in press. [DOI] [PubMed] [Google Scholar]
- 19.Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on α(2)β(1)-integrin expression. J Cell Sci. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- 20.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 21.Richardson GD, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 22.Burger PE, et al. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci USA. 2005;102:7180–7185. doi: 10.1073/pnas.0502761102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goto K, et al. Proximal prostatic stem cells are programmed to regenerate a proximal-distal ductal axis. Stem Cells. 2006;24:1859–1868. doi: 10.1634/stemcells.2005-0585. [DOI] [PubMed] [Google Scholar]
- 25.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh SK, et al. Identification of human brain tumor-initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 28.Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malanchi I, et al. Cutaneous cancer stem cell maintenance is dependent on β-catenin signaling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 30.Fornaro M, et al. Cloning of the gene encoding Trop-2, a cell-surface glycoprotein expressed by human carcinomas. Int J Cancer. 1995;62:610–618. doi: 10.1002/ijc.2910620520. [DOI] [PubMed] [Google Scholar]
- 31.Tsujikawa M, et al. Identification of the gene responsible for gelatinous drop-like corneal dystrophy. Nat Genet. 1999;21:420–423. doi: 10.1038/7759. [DOI] [PubMed] [Google Scholar]
- 32.Wang XD, et al. Expression profiling of the mouse prostate after castration and hormone replacement: Implication of H-cadherin in prostate tumorigenesis. Differentiation. 2007;75:219–234. doi: 10.1111/j.1432-0436.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- 33.El Sewedy T, Fornaro M, Alberti S. Cloning of the murine TROP2 gene: Conservation of a PIP2-binding sequence in the cytoplasmic domain of TROP-2. Int J Cancer. 1998;75:324–330. doi: 10.1002/(sici)1097-0215(19980119)75:2<324::aid-ijc24>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 34.Ohmachi T, et al. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12:3057–3063. doi: 10.1158/1078-0432.CCR-05-1961. [DOI] [PubMed] [Google Scholar]
- 35.Fong D, et al. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–1295. doi: 10.1038/sj.bjc.6604677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang H, et al. Aberrant expression of novel and previously described cell membrane markers in human breast cancer cell lines and tumors. Clin Cancer Res. 2005;11:4357–4364. doi: 10.1158/1078-0432.CCR-04-2107. [DOI] [PubMed] [Google Scholar]
- 37.Tsujimura A, et al. Proximal location of mouse prostate epithelial stem cells: A model of prostatic homeostasis. J Cell Biol. 2002;157:1257–1265. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells. 2007;25:2760–2769. doi: 10.1634/stemcells.2007-0355. [DOI] [PubMed] [Google Scholar]
- 39.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 40.Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 41.Lawson DA, Witte ON. Stem cells in prostate cancer initiation and progression. J Clin Invest. 2007;117:2044–2050. doi: 10.1172/JCI32810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asselin-Labat ML, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 43.Segditsas S, et al. Putative direct and indirect Wnt targets identified through consistent gene expression changes in APC-mutant intestinal adenomas from humans and mice. Hum Mol Genet. 2008;17:3864–3875. doi: 10.1093/hmg/ddn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Day R, Dong Y, Weintraub SJ, Michel L. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol Cancer Ther. 2008;7:280–285. doi: 10.1158/1535-7163.MCT-07-2003. [DOI] [PubMed] [Google Scholar]
- 45.Guerra E, et al. A bicistronic CYCLIN D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism in human cancer. Cancer Res. 2008;68:8113–8121. doi: 10.1158/0008-5472.CAN-07-6135. [DOI] [PubMed] [Google Scholar]
- 46.Baeuerle PA, Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96:417–423. doi: 10.1038/sj.bjc.6603494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada H, et al. Keratin profiles in normal/hyperplastic prostates and prostate carcinoma. Virchows Arch A Pathol Anat Histopathol. 1992;421:157–161. doi: 10.1007/BF01607049. [DOI] [PubMed] [Google Scholar]
- 48.di Sant'Agnese PA. Neuroendocrine differentiation in human prostatic carcinoma. Hum Pathol. 1992;23:287–296. doi: 10.1016/0046-8177(92)90110-o. [DOI] [PubMed] [Google Scholar]
- 49.Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci USA. 2003;100:11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.