Abstract
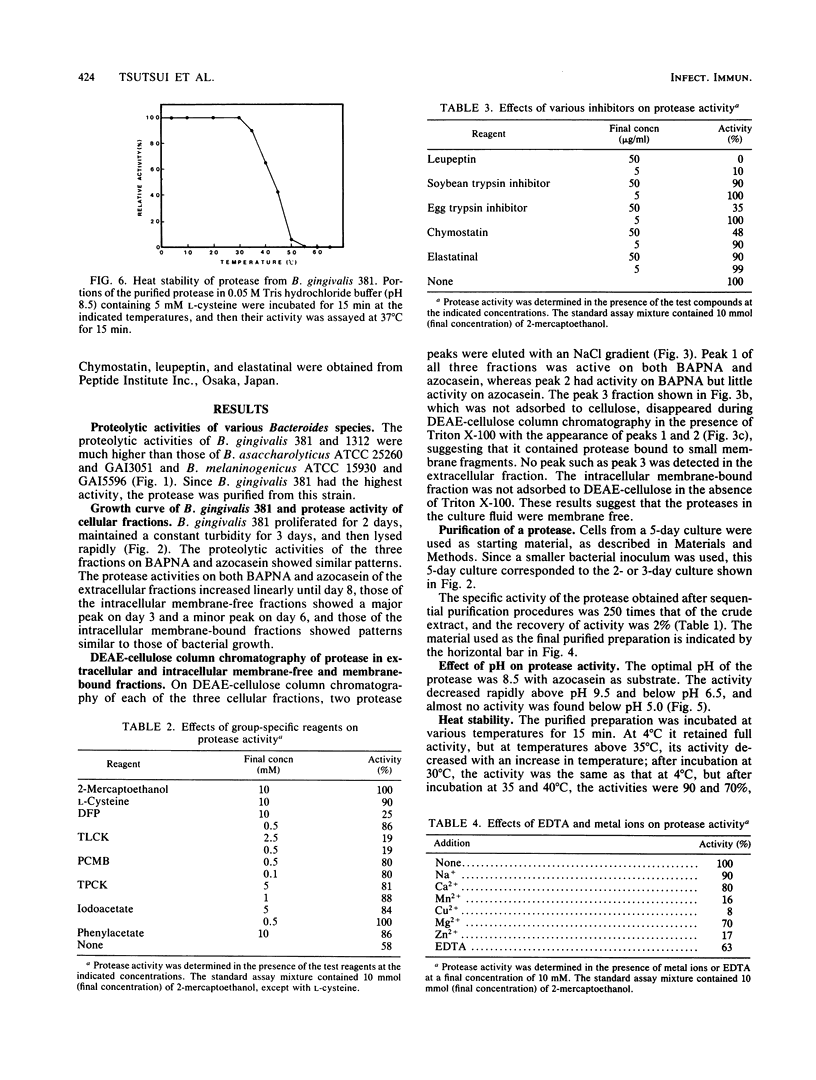
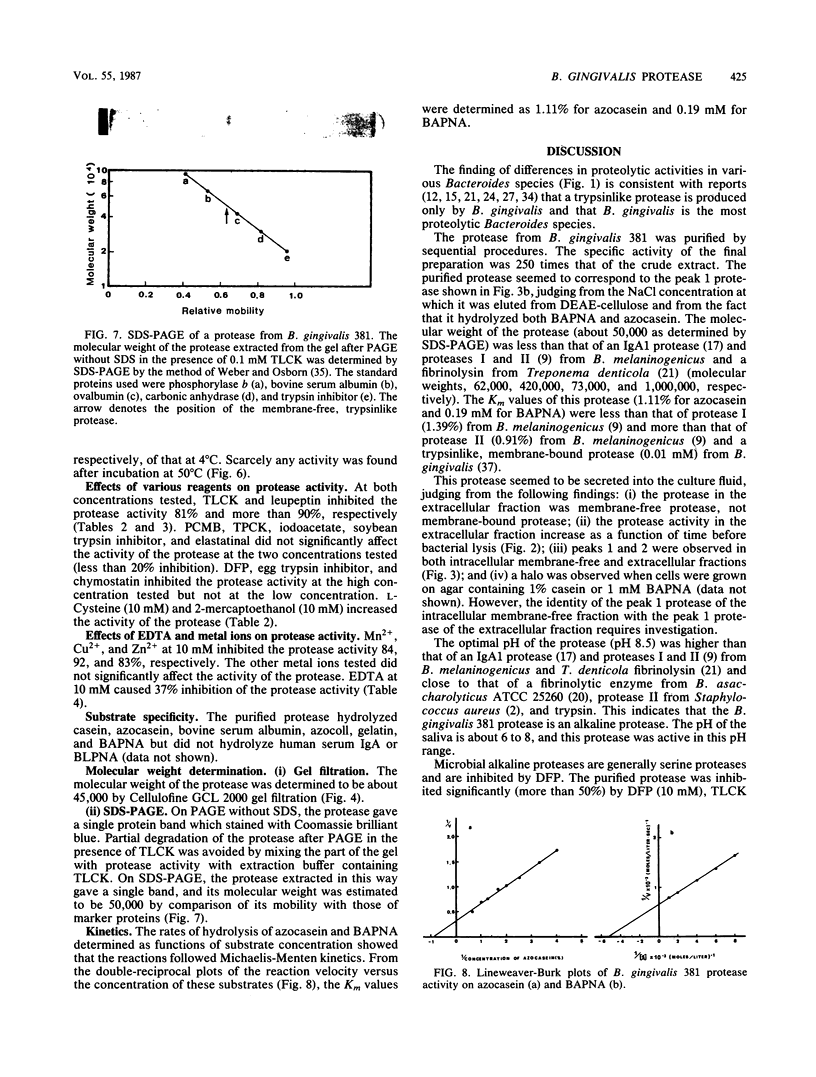
An intracellular membrane-free, trypsinlike protease was isolated from cells of Bacteroides gingivalis 381. The protease was extracted from the cells by ultrasonic treatment and was purified about 250-fold with a recovery of 2% by sequential procedures. The properties of the protease were as follows: its optimal pH was 8.5; its activity was almost completely lost on incubation at 50 degrees C for 15 min; its activity was inhibited by diisopropylfluorophosphate, p-toluenesulfonyl-L-lysine chloromethyl ketone hydrochloride, leupeptin, Mn2+, Cu2+, and Zn2+; it hydrolyzed casein, azocasein, N-alpha-benzoyl-DL-arginine-p-nitroanilide (BAPNA), bovine serum albumin, azocoll, and gelatin, but not N-alpha-benzoyl-DL-lysine-p-nitroanilide or human serum immunoglobulin A; its molecular weight was estimated as 45,000 by gel filtration and 50,000 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis; and its Km values for azocasein and BAPNA were 1.11% and 0.19 mM, respectively.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidson S., Holme T., Lindholm B. Studies on extracellular proteolytic enzymes from Staphylococcus aureus. I. Purification and characterization of one neutral and one alkaline protease. Biochim Biophys Acta. 1973 Mar 15;302(1):135–148. doi: 10.1016/0005-2744(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Arvidson S., Holme T., Wadström T. Influence of cultivation conditions on the production of extracellular proteins by Staphylococcus aureus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):399–405. doi: 10.1111/j.1699-0463.1971.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Boethling R. S. Purification and properties of a serine protease from Pseudomonas matophilia. J Bacteriol. 1975 Mar;121(3):933–941. doi: 10.1128/jb.121.3.933-941.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Zipser D. Purification and characterization of protease III from Escherichia coli. J Biol Chem. 1979 Jun 10;254(11):4698–4706. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R., Boily Y., Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem. 1972 Oct 25;247(20):6720–6726. [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Isolation and characterization of proteases from Bacteroides melaninogenicus. Infect Immun. 1981 Sep;33(3):738–742. doi: 10.1128/iai.33.3.738-742.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. Degradation of immunoglobulins A2, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981 Dec;34(3):757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., Grenier D. Detection of collagenase activity in oral bacteria. Can J Microbiol. 1985 Feb;31(2):134–138. doi: 10.1139/m85-026. [DOI] [PubMed] [Google Scholar]
- Mayrand D., McBride B. C. Exological relationships of bacteria involved in a simple, mixed anaerobic infection. Infect Immun. 1980 Jan;27(1):44–50. doi: 10.1128/iai.27.1.44-50.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen S. B., Kilian M. Purification and characterization of an immunoglobulin A1 protease from Bacteroides melaninogenicus. Infect Immun. 1984 Sep;45(3):550–557. doi: 10.1128/iai.45.3.550-557.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977 Mar;12(2):120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Nilsson T., Carlsson J., Sundqvist G. Inactivation of key factors of the plasma proteinase cascade systems by Bacteroides gingivalis. Infect Immun. 1985 Nov;50(2):467–471. doi: 10.1128/iai.50.2.467-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan D., Sperry J. F., Wilkins T. D. Fibrinolytic activity of oral anaerobic bacteria. Arch Oral Biol. 1978;23(6):465–470. doi: 10.1016/0003-9969(78)90078-x. [DOI] [PubMed] [Google Scholar]
- Robertson P. B., Lantz M., Marucha P. T., Kornman K. S., Trummel C. L., Holt S. C. Collagenolytic activity associated with Bacteroides species and Actinobacillus actinomycetemcomitans. J Periodontal Res. 1982 May;17(3):275–283. doi: 10.1111/j.1600-0765.1982.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Ross E., Schatz G. Assay of protein in the presence of high concentrations of sulfhydryl compounds. Anal Biochem. 1973 Jul;54(1):304–306. doi: 10.1016/0003-2697(73)90280-7. [DOI] [PubMed] [Google Scholar]
- Rudek W., Haque R. U. Extracellular enzymes of the genus Bacteroides. J Clin Microbiol. 1976 Nov;4(5):458–460. doi: 10.1128/jcm.4.5.458-460.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH H. L., Jr, GOODNER K. Detection of bacterial gelatinases by gelatin-agar plate methods. J Bacteriol. 1958 Dec;76(6):662–665. doi: 10.1128/jb.76.6.662-665.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Enzymatic characterization of some oral and nonoral gram-negative bacteria with the API ZYM system. J Clin Microbiol. 1981 Sep;14(3):288–294. doi: 10.1128/jcm.14.3.288-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979 Oct;6(5):351–382. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S. Relationship of bacteria to the etiology of periodontal disease. J Dent Res. 1970 Mar-Apr;49(2):203–222. doi: 10.1177/00220345700490020401. [DOI] [PubMed] [Google Scholar]
- Spiegel C. A., Hayduk S. E., Minah G. E., Krywolap G. N. Black-pigmented Bacteroides from clinically characterized periodontal sites. J Periodontal Res. 1979 Sep;14(5):376–382. doi: 10.1111/j.1600-0765.1979.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Takazoe I., Tanaka M., Homma T. A pathogenic strain of Bacteroides melaninogenicus. Arch Oral Biol. 1971 Jul;16(7):817–822. doi: 10.1016/0003-9969(71)90126-9. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., Reynolds H. S., Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect Immun. 1981 Apr;32(1):198–203. doi: 10.1128/iai.32.1.198-203.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenbergen T. J., Kastelein P., Touw J. J., de Graaff J. Virulence of black-pigmented Bacteroides strains from periodontal pockets and other sites in experimentally induced skin lesions in mice. J Periodontal Res. 1982 Jan;17(1):41–49. doi: 10.1111/j.1600-0765.1982.tb01129.x. [DOI] [PubMed] [Google Scholar]