Abstract
Background:
In utero exposure to smoking is known to adversely affect brain regions involved in behavioral state organization and could therefore interact with the neurophysiological development of neonates.
Study Objectives:
The present study investigated the effects of prenatal smoking exposure on sleep patterns in the preterm neonate.
Design:
Overnight sleep patterns were polysomnographically assessed at thermoneutrality. Sleep continuity and structure were scored for the respective frequencies, durations and percentages of active, quiet, and indeterminate sleep and wakefulness after sleep onset. The number and duration of body movements were also analyzed.
Setting:
The neonatal intensive care unit at Amiens University Medical Center (France).
Participants:
Healthy preterm neonates (postconceptional age: 33.9 ± 6.0 weeks) were enrolled according to whether their mothers had not smoked at all during pregnancy (control group, n = 19), smoked less during pregnancy (low-smoking group, Slow, n = 10), or smoked more (heavy-smoking group, Sheavy, n = 10) than 10 cigarettes per day throughout pregnancy.
Measurements and Results:
Neonates born to heavy-smoking mothers had a significantly lower mean birth weight than controls (–21%) and displayed disrupted sleep structure and continuity: they slept less overall (with a higher proportion of active sleep and a lower proportion of quiet sleep) and had more wakefulness after sleep onset. Compared with controls, neonates from both smoking groups displayed more body movements and, as a result, more disturbed sleep.
Conclusions:
High prenatal smoking exposure modifies sleep patterns in preterm neonates by disrupting sleep organization and increasing nocturnal body movements. These findings raise the question of the repercussions of these sleep disturbances (at what is a critical stage in brain development) on the child's physiological and neurobehavioral outcomes.
Citation:
Stéphan-Blanchard E; Telliez F; Léké A; Djeddi D; Bach V; Libert JP; Chardon K. The influence of in utero exposure to smoking on sleep patterns in preterm neonates. SLEEP 2008;31(12):1683–1689.
Keywords: Preterm neonates, Prenatal smoking exposure, Sleep structure, Body movements
NEONATES SPEND THE MAJOR PART OF THEIR EARLY LIFE ASLEEP. THE EARLY DEVELOPMENTAL PERIOD FEATURES MAJOR CHANGES IN SLEEP PATTERNS, reflecting the degree of brain maturation and plasticity. Sleep-wake state integrity is also of paramount importance in the neurophysiological development (e.g., body growth and vital functions) of neonates in general1,2 and premature neonates in particular.
Several studies have reported that maternal cigarette smoking during pregnancy may have harmful effects on fetal development.3,4 Cigarettes are known to deliver large amounts of chemical toxins to the fetus via the maternal bloodstream and increase the norepinephrine and dopamine concentrations in the central catecholaminergic systems of the developing brain.5 This can have potentially negative effects (such as cell damage and disrupted development) on the central nervous system.6 The brain regions involved in these smoking-related changes participate in neurobehavioral functions such as sleep-wake state organization and somatic motor control.7,8
In human adults, it has been previously reported that smokers have trouble falling asleep and suffer from poor sleep maintenance (and thus greater intra-sleep wakefulness).9 Moreover, it has often been reported that both acute10 and chronic11 exposure to nicotine have adverse effects on sleep structure, even though the effects of nicotine depend on the dose and the administration route. Prenatal smoking exposure is also highly related to sudden infant death syndrome (SIDS). To improve the understanding of SIDS mechanisms, a number of studies have examined the effects of in utero exposure to smoking on autonomic functions during sleep in newborn infants. Although these studies pointed out significant effects of maternal smoking on arousal,12–14 autonomic nervous system activity15 or apnea,16 they did not find any influence of prenatal smoking exposure on sleep structure. However, in two relatively old studies, Longo17 and Fried et al18 observed hyperactivity or hypertonicity in neonates following prenatal exposure to tobacco. Likewise, frequent physical and behavioral effects of prenatal exposure to nicotine have been observed in various animal species.19,20 Taken as a whole, these studies show that in utero exposure to nicotine is associated with increased motor activity. Franck et al21 also found that prenatal nicotine exposure profoundly altered the sleep-wake cycle of rat pups: total sleep time (TST) decreased as a result of increased awakening and decreased REM sleep.
The above observations emphasize the fact that despite the crucial role of sleep in neurophysiological development, there is a lack of data on the effects of prenatal smoking exposure on sleep patterns in human neonates. The available literature is heterogeneous and partly conflicting. The goal of the present study was to assess the influence of in utero exposure to smoking on sleep patterns in the developing preterm neonate. Specifically, we examined whether prenatal smoking exposure altered sleep structure and stability and motor activity.
MATERIAL AND METHODS
Infants
Forty healthy preterm neonates (means ± SD, gestational age: 31.3 ± 1.7 weeks, postconceptional age: 33.9 ± 6.0 weeks) were recruited from the neonatal intensive care unit (NICU) at Amiens University Medical Center (France) after their parents had been informed of the protocol and had given their written consent. The protocol was approved by the Picardy Regional Institutional Review Board. All neonates were free of neurological, respiratory, and cardiac disorders and had not received caffeine treatment or oxygen therapy. Some infants received mechanical ventilation via endotracheal tube, but no studied infant had been on ventilatory support for at least 7 days before the study. Recordings were performed after determining that there had been no changes in health status in previous days. None of the infants had suffered from sleep deprivation in the 48 hours prior to the sleep recording session.
In order to determine the level of in utero exposure to smoking, mothers were asked to evaluate the daily number of cigarettes that they had smoked during pregnancy. Even if various studies have pointed out that such self-reports are acceptably reliable,22,23 the results remain dependent on the subjective report of the mother. The mothers' medical records were reviewed, paying special attention to any history of smoking during pregnancy for comparison with the self-report. A short questionnaire was used to acquire additional information; neonates whose mothers reported substance abuse or passive smoking were excluded from the study. On the basis of the questionnaire responses, 3 homogeneous groups of neonates (Table 1) were constituted, according to whether their mothers had not smoked during pregnancy (control group), smoked <10 cigarettes per day (low-smoking group, Slow), or smoked >10 cigarettes per day (heavy-smoking group, Sheavy) throughout pregnancy. To preserve the homogeneity among the 3 groups of neonates, the data of one infant in the Sheavy group were discarded from analyses based on a very low birth weight history and a significantly older postnatal age at the time of the recording (87 days).
Table 1.
Clinical Parameters for the 3 Groups of Neonates
| Control group n = 19 | Slow group n = 10 | Sheavy group n = 10 | |
|---|---|---|---|
| Cigarettes / day | 0 | 4.9 (1–9) *** | 16.2 (10–25) ***††† |
| Gestational age, weeks | 31.7 (29.1–34.4) | 31.2 (28.6–34.1) | 31.2 (27.8–33.3) |
| Birth weight, g | 1675 (1080–2180) | 1488 (850–2190) | 1325 (949–2074) * |
| Postnatal age, days | 19.8 (1–48) | 20.2 (0–61) | 29.7 (0–61) |
| Postconceptional age, weeks | 34.5 (30.3–36.9) | 34.1 (29.1–38.7) | 35.7 (32.0–37.7) |
| Weight at the time of the study, g | 1975 (1020–2525) | 1807 (963–2810) | 1997 (1010–2635) |
| Mechanical ventilation, days | 3.4 (0–6) | 3.8 (0–6) | 5.1 (0–8) |
| Arterial oxygen saturation, % | 97.6 (96.4–98.3) | 97.9 (96.5–99.0) | 97.4 (96.3–97.9) |
| Respiratory rate, breaths/min | 52.1 (44.2–61.4) | 52.0 (39.7–63.4) | 54.6 (45.5–61.1) |
| Heart rate, beats/min | 146 (137–161) | 150 (135–166) | 146 (133–167) |
Values are means and ranges.
P < 0.05
P < 0.001 versus control group
P < 0.001 versus Slow group.
As breastfeeding during the early postnatal period has been found to reduce some of the adverse effects of prenatal smoking exposure on sleep,24 and as differences in the method of feeding or in feeding formulas have been found to modify sleep patterns,25 neonates of the control, Slow and Sheavy groups were all recruited from the NICU among mothers who did not breastfeed their infants. Infants were not exposed to environmental tobacco smoke after birth.
Polysomnographic Recordings
Overnight polysomnographic recordings were made between 19:00 on day 1 and 08:00 on day 2, and began just after a feed. The infants slept in a separate room, i.e., isolated from routine nursing activities, noise, and light changes. Neonates were nursed in a closed, convectively heated incubator (Médipréma ISIS, Chambray-les-Tours, France) at thermoneutrality, in order to avoid any thermal effect on sleep patterns.26 The room's air temperature was kept constant at 22°C (relative air humidity: 30% to 40%, air velocity ≤0.04 m/sec, natural convection). Neonates wore only a small diaper and were lying in the supine position on a mattress.
Electrophysiological recordings included right and the left centro-occipital leads electroencephalographic leads (EEG), an electrocardiogram (ECG) and respiratory movement monitoring (assessed by transthoracic impedance using the ECG patch electrodes). Eye movements were monitored by a piezoelectric quartz transducer attached to an eyelid. Body movements were recorded by accelerometers attached to a wrist and the opposite ankle. Transcutaneous arterial oxygen saturation values were recorded with a pulse oximeter (Oximax MAX-N, Tyco Healthcare group LP, Nellcor Puritan Bennett Division, CA). All recordings were continuously monitored on a polysomnogram (Alice 4, Respironics, Nantes, France).
Sleep states were scored visually in 30-sec periods, according to Curzi-Dascalova and Mirmiran.27 In addition, infants were video-recorded throughout the night. Although video monitoring was not used to score sleep states, it helped to exclude nursing interventions and feeding episodes from the analysis. The scorers were blinded to the infant's designated study group and the study hypothesis. Sleep states were differentiated on the basis of concordance between EEG and REM signals. As necessary, breathing parameters were superimposed on these measurements to help define states. Wakefulness was defined as a state in which the infant's eyes were open (whether scanning the environment or not) and when frequent body movements occurred. Crying or fussing was sometimes observed. Active sleep (AS) was defined as continuous EEG activity with REM, while quiet sleep (QS) was defined as discontinuous EEG activity without REM. Periods that did not meet the criteria for either AS or QS (i.e., discontinuous EEG with REM or [most commonly] continuous EEG without REM) were defined as indeterminate sleep (IS).
Data Analysis
The temporal organization of sleep states falls into 2 categories:28 sleep structure and sleep stability, i.e., the ability to maintain sleep. The following criteria were taken into account: sleep period time (SPT), beginning at the first sleep onset and ending at the last awakening; the percentage and frequency of wakefulness after sleep onset (WASO), expressed as a proportion of the SPT; total sleep time (TST), defined as the difference between SPT and WASO durations; and the percentage and frequency of the different sleep states, expressed as a proportion of TST. The duration of the longest QS episode, which may be related to the development and maturation of the central nervous system of the neonate,29 was also analyzed.
Special attention was paid to the number and duration of body movements. All active periods separated by <1 sec were regarded as a single movement. Conversely, motor activity lasting <1 sec but within a quiet period was not considered to be a movement. Nocturnal awakening episodes with frequent body movements were not taken into account in the analysis. The temporal structure of movements was evaluated by measuring the frequency and the total duration of body movements (TDM), expressed relative to the TST and to each sleep state.
Statistical Analysis
Statistics were computed using Statview software (version 5.0, SAS Institute Inc., Cary, NC). Normal data distribution was checked using a Kolmogorov-Smirnov test. One-way analyses of variance (ANOVA), with group as the between-subject factor were used to test differences in sleep structure and stability parameters. Body movement parameters were analyzed using 2-way ANOVAs, with sleep state as the within-subject factor and group as the between-subject factor. Subscripts beneath F values represent the degrees of freedom. When F values were significant, paired or unpaired t-tests were computed. Values expressed as percentages were arcsine transformed in order to stabilize the variance.30 The significance threshold P was set to < 0.05. Data are given as means ± 1 SD.
RESULTS
Infants
Table 1 shows that the greater in utero exposure to smoking was associated with lower birth weight. Compared with non-exposed (control) neonates, prenatal smoking exposure decreased birth weight by 11% in the Slow group and 21% in the Sheavy group, although the difference was only significant in the latter (P = 0.020). By the time of the study, intergroup differences in body weight were no longer observed. This could be due (at least in part) to the fact that polysomnographic recordings in neonates in the Sheavy group were performed at a greater postnatal age, even though the difference in this latter parameter was not statistically significant.
The time spent under mechanical ventilation did not differ significantly between the control, Slow and Sheavy groups. Prenatal smoking exposure did not modify the mean blood oxygen saturation, or heart or respiratory rates during the nocturnal polysomnographic recordings. Heart and respiratory rates were higher in AS than in QS in all groups of neonates (heart rate: 152 ± 11 and 145 ± 9 beats/min, respectively, P < 0.001; respiratory rate: 58.3 ± 9.7 and 49.1 ± 7.2 breaths/min, respectively, P = 0.002).
Effects of Prenatal Smoking Exposure on Sleep Organization
The mean duration of recording was identical in the 3 groups (11.4 ± 1.1 h). All the neonates had well-defined sleep-wake cycles. In utero exposure to smoking modified both sleep stability (Table 2) and structure (Figure 1). These effects were only observed in neonates born to heavy-smoking mothers (Sheavy group).
Table 2.
Effects of In Utero Exposure to Smoking on Sleep Stability Parameters
| Control group | Slow group | Sheavy group | Group effect (F; P values) | |
|---|---|---|---|---|
| SPT, min | 677 ± 50 | 667 ± 37 | 701 ± 67 | NS |
| TST, min | 622 ± 65 | 587 ± 35 | 510 ± 58 | F2,36 = 12.4; P < 0.001 |
| WASO, % SPT | 8 ± 7 | 12 ± 4 | 27 ± 4 | F2,36 = 41.4; P < 0.001 |
| f WASO/h | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.7 ± 0.2 | F2,36 = 3.6; P = 0.037 |
| f SSC/h | 2.4 ± 0.7 | 2.7 ± 0.7 | 2.8 ± 0.8 | NS |
| AS, min | 416 ± 44 | 367 ± 57 | 399 ± 90 | NS |
| QS, min | 150 ± 65 | 156 ± 37 | 79 ± 31 | F2,36 = 7.4; P = 0.002 |
| IS, min | 56 ± 52 | 64 ± 32 | 32 ± 27 | NS |
| Longest AS episode, min | 34.1 ± 12.2 | 32.2 ± 10.6 | 38.7 ± 13.8 | NS |
| Longest QS episode, min | 20.5 ± 7.5 | 23.4 ± 9.1 | 14.6 ± 4.3 | F2,36 = 3.8; P = 0.032 |
Values are given as means ± SD.
NS: non-significant; SPT: sleep period time; TST: total sleep time; WASO: wakefulness after sleep onset; f WASO: frequency of wakefulness after sleep onset episodes; f SSC: frequency of sleep-wake state changes; AS, IS, and QS: active, indeterminate, and quiet sleep.
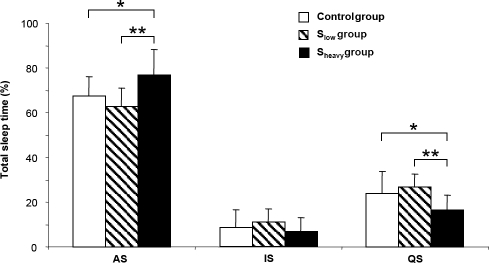
Figure 1.
Mean (± SD) active (AS), quiet (QS) and indeterminate (IS) sleep expressed as a percentage of total sleep time in neonates in control, low-smoking (Slow) and heavy-smoking (Sheavy) groups. *P < 0.05; **P < 0.01.
The 3 groups did not differ in terms of SPT. Although in utero exposure to smoking did not modify the overall frequency of sleep-wake state changes, neonates in the Sheavy group showed an increase in the frequency of WASO episodes (P = 0.019 vs. controls) and in the amount of WASO relative to SPT (P < 0.001 vs. controls). As a result, TST was lower in the Sheavy group than in the control group (−18%, P < 0.001).
The amount of QS (in min) was lower in the Sheavy group (P = 0.003 vs. controls; P < 0.001 vs. Slow). Moreover, neonates in the Sheavy group showed shorter duration of the longest QS episode than controls (P = 0.030) and neonates in the Slow group (P = 0.013). This was not found for the longest AS episode. As a result, the relative duration of AS was higher in the Sheavy group than in either the control group (+15%, P = 0.012) or the Slow group (+24%, P = 0.004) and was reflected by a decrease in the percentage of QS (−34% vs. controls, P = 0.024; −41% vs. Slow, P = 0.001). There were no differences among the 3 groups in terms of the percentage of IS.
Effects of Prenatal Smoking Exposure on Motor Activity
Variables describing body movements for the 3 groups of neonates in the different sleep stages are shown in Table 3. In all groups of neonates, the TDM (expressed as a percentage of time in each sleep stage) was higher in AS than in IS, and higher in IS than in QS (10.3% ± 4.4%, 7.3% ± 4.3% and 5.6% ± 4.2%, respectively; F2,36 = 917.3; P < 0.001; each comparison was statistically significant). A similar result was found for the frequency of body movement (0.99 ± 0.38, 0.94 ± 0.38 and 0.89 ± 0.37/min, respectively; F2,36 = 77.7; P < 0.001).
Table 3.
Effects of In Utero Exposure to Smoking on Sleep Body Movement Parameters
| Control group | Slow group | Sheavy group | Group effect (F; P values) | |
|---|---|---|---|---|
| Total duration of body movement, % | ||||
| AS | 7.9 ± 2.3 | 11.7 ± 3.6 | 13.3 ± 5.8 | F2,36= 7.7; P = 0.002 |
| IS | 4.9 ± 2.1 | 8.8 ± 3.5 | 10.6 ± 5.5 | F2,36 = 9.6; P < 0.001 |
| QS | 3.3 ± 1.9 | 6.8 ± 3.6 | 8.6 ± 5.7 | F2,36 = 7.8; P = 0.001 |
| All sleep states | 4.9 ± 2.1 | 8.8 ± 3.5 | 10.6 ± 5.4 | F2,36 = 9.5; P ≤ 0.001 |
| Body movement frequency, min−1 | ||||
| AS | 0.81 ± 0.31 | 1.09 ± 0.40 | 1.22 ± 0.34 | F2,36 = 5.2; P = 0.010 |
| IS | 0.76 ± 0.30 | 1.06 ± 0.41 | 1.17 ± 0.34 | F2,36 = 5.5; P = 0.008 |
| QS | 0.71 ± 0.31 | 1.00 ± 0.39 | 1.10 ± 0.32 | F2,36 = 5.3; P = 0.010 |
| All sleep states | 0.77 ± 0.31 | 1.05 ± 0.40 | 1.16 ± 0.34 | F2,36 = 5.1; P = 0.011 |
Values are given as means ± SD.
AS: active sleep; IS: indeterminate sleep; QS: quiet sleep.
In utero exposure to smoking increased significantly the TDM: the control group spent less time moving than the Slow group (P < 0.001) or the Sheavy group (P < 0.001). Similar results were found for the frequency of occurrence of body movements: the control group's sleep was less disturbed by body movements than that of the Slow group (P = 0.043) or the Sheavy group (P = 0.004). No difference between neonates from low-smoking mothers and neonates from heavy-smoking mothers was observed in this respect.
DISCUSSION
The present study deals with the influence of in utero exposure to smoking on sleep patterns in preterm neonates. We chose to investigate sleep structure and stability and body activity during sleep because these parameters reflect brain maturation and provide information on the neuronal control of physiological and behavioral functions in neonates. In the present study, the control group of neonates yielded similar sleep structure and body activity data to those previously recorded in our laboratory31 or by other investigators32 in neonates of similar postconceptional age.
To the best of our knowledge, this study is the first to show that high levels of prenatal smoking exposure strongly modify sleep patterns in preterm neonates. The present data demonstrate altered sleep structure (decreased TST and QS proportion, increased AS proportion) and higher sleep fragmentation (greater frequency of WASO and sustained body movements) in preterm neonates born to heavy-smoking mothers. The results need to be interpreted with caution, as neonates in the Sheavy group were studied at a greater postnatal age (nearly 10 days) than the others, even though this difference was not statistically significant. As a consequence, some differences in sleep and body activity parameters attributed to prenatal exposure to heavy smoking may have been influenced by either brain maturation or more time spent in the NICU in the Sheavy group.
However, the difference in sleep patterns between the control and Sheavy groups can not easily be attributed to more stressful experiences in the first days of life (nursing interventions, environmental factors) since medical history (oxygen therapy, mechanical ventilation) and treatments (caffeine) did not differ among the 3 groups of neonates. Infants in the Sheavy group spent more time in the NICU than controls, but the effects of heavy prenatal smoking exposure on sleep patterns are the opposite of those observed with care activity stimulations. Such stimulations inevitably induce more short periods of sleep deprivation, leading to compensatory increases in sleep time and/or intensity during recovery. Moreover, neonates respond to selective or total sleep deprivation by compensatory increases in QS time only.33,34
Secondly, the difference in sleep patterns between the control and Sheavy groups would reflect the effect of advancing age rather than the effect of prenatal smoking exposure. It is commonly established35 that the early development of sleep is characterized by a rapid increase in the proportion of QS while AS remains fairly constant. The development continues during the first year, with an increasing proportion of QS, mainly at the expense of AS. Moreover, nocturnal sleep continuity increases with advancing age, as a consequence of a concomitant increase in sleep state duration and decreased the frequency of nocturnal wakefulness. Once again, the present results on sleep patterns in neonates in the Sheavy group go in an opposite way than those of advancing age. Hence, the difference in sleep patterns between the control and Sheavy groups can not be attributed to their difference in postnatal age, and even suggests an underestimation of the effect of heavy prenatal smoking exposure on sleep patterns.
Our findings are consistent with literature reports of the teratogenic effect of nicotine on the developing central nervous system. Nicotine produces adverse signaling in brain regions such as the reticular activating system, which is involved in the control of activity, arousal, waking and REM sleep. Prenatal smoking exposure can induce up-regulation of nicotinic receptors in the brain, resulting in greater release of dopamine, norepinephrine, serotonin, and acetylcholine.5 Nicotine's excitatory effects on the reticular activating system could thus disrupt normal sleep processes and result in greater activity, arousal, and REM sleep.
The neurotoxic effects of in utero exposure to smoking with respect to sleep-wake properties have not been thoroughly studied, and the available data are partly conflicting. Our results for nocturnal body movements are consistent with previous studies, which reported greater motor activity in both human neonates17,18 and animal species19,20 exposed in utero to nicotine. In contrast, our findings on sleep structure disagree with most other studies in human neonates, which have reported a lack of sleep structure differences between controls and infants exposed prenatally to maternal smoking.12–16 However, as mentioned above, none of these studies were specifically designed to assess this relationship, and the disparity of the methodologies does not enable confirmation of the absence of influence of prenatal smoking exposure. Some studies assessed the influence of prenatal smoking exposure on infant arousability, so that the sleep structure would have been disturbed by the arousal stimuli.12–14 In other studies, the sleep analysis pooled data from neonates of 0.5 weeks postnatal age with those from 11-week-old children,15,16 and it was not always possible even to distinguish whether the effects of smoking occurred prenatally or postnatally.16 Nevertheless, an alteration in vigilance state organization has been found in newborn rats exposed prenatally to nicotine (2 or 4 mg/kg/day).21 Although these authors reported a significant reduction in REM sleep following prenatal nicotine exposure (in contrast to our present findings), they also found less total sleep and more waking.
Altered sleep organization may have physiological significance. Initial evidence is provided by the disruption of sleep stability, with a decrease in TST and increases in the frequency of WASO and nocturnal body movements. Maternal smoking during pregnancy increases the risk of SIDS, and the relationship between SIDS and prenatal smoking exposure appears to be dose-dependent.36 Strikingly, prenatal smoking exposure produced sleep pattern abnormalities that contrast with those found in infants at risk of SIDS since one suggested etiology for SIDS involves alterations in both stimulus-induced and spontaneous arousal from sleep in infants exposed prenatally to tobacco smoke.37 This comparison requires caution since preterm infants studied in NICU may not have the same sleep stability (stressful environment), as infants born at term and living at home at the time of the study.37 Indeed, routine care procedures could not be completely avoided; and handling, noise, and lightning in the NICU are well-known factors that increase arousals or sleep state transitions.38 The results of the present study suggest, however, that if prenatal smoking exposure does increase the risk of SIDS, one possible pathophysiological mechanism may involve an absence of organized, central control (when required), rather than a decrease in the ability to arouse from sleep.
Sleep maintenance and stability are crucial for growth and brain development, especially in preterm neonates. The period from 34 to 40 weeks of postmenstrual age is a developmental window during which neurobehavioral systems (e.g., sleepwake states and body movements) undergo a transition into an organized neonatal pattern, with well-defined sleep cycles.39 Thus, the effects observed here testify to the difficulty of the exposed infants of organizing and maintaining their sleep, probably as a consequence of a comparative immaturity. The fact that the duration of the longest QS episode was significantly shorter in neonates of the Sheavy group argues in favor of this hypothesis because QS is a highly controlled sleep stage, which could be representative of the development of cortical control.29 Moreover, an increase in nocturnal body movements and a decrease in TST may have deleterious effects (caused by long-term sleep deprivation) and could place the exposed infants at a higher risk of a negative developmental outcome.
The second aspect relates to the disruption of sleep structure in significantly smoke-exposed preterm neonates, who show greater percentages of WASO and AS and a corresponding reduction in QS, compared with controls. A markedly altered postnatal sleep pattern observed in neonates whose mothers smoked during pregnancy also suggests suboptimal central nervous system organization and could thus explain disrupted regulation of autonomic and vegetative functions, which are known to interact during sleep. It is commonly assumed that both cardiac and respiratory functions vary according to the sleep state. In AS, heart rate variability and breathing are always irregular, and central apnea occurs very frequently.40 Moreover, previous studies from our group have shown that chemical control of breathing is sleep state dependent.41 The high amount of AS observed in this study could increase the risk of cardiorespiratory disorders during sleep, since exposed infants might present less stable breathing and a greater incidence of cardiorespiratory challenges (e.g., apnea). It has been suggested that modifications in sleep-wake ontogeny may trigger SIDS events by producing misalignments between vegetative functions that normally become integrated during the postnatal period, thus leading to the failure of homeostatic processes.42
Our findings indicate that even after 29.7 days without postnatal exposure to smoking or nicotine, and despite the fact that neonates in the Sheavy group were nearly 10 days older, infants exposed in utero to high levels of smoking still showed an altered organization of the various behavioral states. These data add to a growing body of literature that shows that prenatal exposure to neurodevelopmental teratogens may have long-lasting, postnatally apparent effects. Moreover, long-term cognitive disorders during childhood and adolescence have been demonstrated. For example, prenatal smoking exposure can lead to deficits in sustained attention and impulsivity in adolescence43 and a higher risk of attention deficit hyperactivity disorder in childhood44—effects that could be mediated (at least in part) by sleep changes. Indeed, studies have demonstrated that sleep disturbances in preterm infants are related to the same later neurodevelopmental outcomes found after prenatal smoking exposure.45,46 Thus, it is important to examine the longevity of the observed differences in brain activity. The developing brain is known for its plasticity and ability to reorganize in response to stimulation provided by the postnatal environment. Consequently, it is possible that intergroup differences may become smaller over the course of development. Longitudinal studies are necessary to assess the persistence of behavioral state effects caused by prenatal smoking exposure.
In conclusion, in utero exposure to heavy smoking was found to modify sleep patterns in preterm neonates. Our data evidenced a concomitant disruption in sleep organization and an increase in nocturnal body movements. Abnormal sleep processes may alter compensatory responses to autonomic cardiovascular/respiratory challenge and increase the likelihood of life-threatening events later in life. Although more research is needed, it is clear that smoking during pregnancy places infants at a higher risk of developmental difficulties which could persist throughout early and middle childhood. Therefore, examining the neurodevelopmental trajectories of neonates exposed to maternal smoking (and of those who were not) could lead to greater understanding of potential deficits in the exposed group, better prediction of outcomes and, potentially, more effective compensatory clinical interventions.
ACKNOWLEDGMENTS
The authors thank S. Delanaud, M.C. Godefroy, and the staff of the Neonatology Department for their assistance with the experiments. They also wish to thank D. Fraser for revising the article's English.
Financial support: This work was funded by grants from the French Ministry of Research.
ABBREVIATIONS
- AS
Active sleep
- ECG
Electrocardiogram
- EEG
Electroencephalogram
- f
Frequency (/min)
- IS
Indeterminate sleep
- NICU
Neonatal intensive care unit
- NS
Non-significant
- QS
Quiet sleep
- REM
Rapid eye movement
- Slow
Neonates born to mothers in the low-smoking group
- Sheavy
Neonates born to mothers in the heavy-smoking group
- SIDS
Sudden infant death syndrome
- SPT
Sleep period time (min)
- TDM
Total duration of body movements (%)
- TST
Total sleep time (min)
- WASO
Wakefulness after sleep onset
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Curzi-Dascalova L, Challamel MJ. Neurophysiological basis of sleep development. In: Loughlin GM, Carroll JL, Marcus CL, editors. Sleep and breathing in children: a developmental approach. New York: Marcel Dekker; 2000. pp. 3–37. [Google Scholar]
- 2.Mirmiran M, Ariagno R. Role of REM sleep in brain development and plasticity. In: Maquet P, Smith C, Stickgold R, editors. Sleep and brain plasticity. Oxford: Oxford University Press; 2003. pp. 181–8. [Google Scholar]
- 3.Hellstrom-Lindahl E, Nordberg A. Smoking during pregnancy: a way to transfer the addiction to the next generation? Respiration. 2002;69:289–93. doi: 10.1159/000063261. [DOI] [PubMed] [Google Scholar]
- 4.Lassen K, Oei TP. Effects of maternal cigarette smoking during pregnancy on long-term physical and cognitive parameters of child development. Addict Behav. 1998;23:635–53. doi: 10.1016/s0306-4603(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 5.Oliff HS, Gallardo KA. The effect of nicotine on developing brain catecholamine systems. Front Biosci. 1999;4:883–97. doi: 10.2741/oliff. [DOI] [PubMed] [Google Scholar]
- 6.Zheng JQ. Turning of the nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–4. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 7.Kinney HC, O'Donnal TJ, Kriger P, White WS. Early developmental changes in [3H] nicotine binding in human brainstem. Neuroscience. 1993;55:1127–30. doi: 10.1016/0306-4522(93)90326-b. [DOI] [PubMed] [Google Scholar]
- 8.Robbins TW. Arousal systems and attentional processes. Biol Psychol. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Samet J, Caffo B, Punjabi NM. Cigarette smoking and nocturnal sleep architecture. Am J Epidemiol. 2006;164:529–537. doi: 10.1093/aje/kwj231. [DOI] [PubMed] [Google Scholar]
- 10.Davila DG, Hurt RD, Offord KP, Harris CD, Shepard JW., Jr. Acute effects of transdermal nicotine on sleep architecture, snoring, and sleep-disordered breathing in nonsmokers. Am J Respir Care Med. 1994;150:469–74. doi: 10.1164/ajrccm.150.2.8049831. [DOI] [PubMed] [Google Scholar]
- 11.Gillin JC, Lardon M, Ruiz C, Golshan S, Salin-Pascual RJ. Dose-dependent effects of transdermal nicotine on early morning awakening and rapid eye movement sleep time in non-smoking normal volunteers. J Clin Psychopharmacol. 1994;14:264–7. [PubMed] [Google Scholar]
- 12.Horne RSC, Ferens D, Watts AM, et al., editors. Maternal tobacco smoking impairs arousal in healthy term infants sleeping supine. Arch Dis Child Fetal Neonatal. 2002;87:F100–F105. doi: 10.1136/fn.87.2.F100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco P, Groswasser J, Hassid S, Lanquart JP, Scaillet S, Kahn A. Prenatal exposure to cigarette smoking associated with a decrease in arousal in infants. J Pediatr. 1999;135:34–8. doi: 10.1016/s0022-3476(99)70324-0. [DOI] [PubMed] [Google Scholar]
- 14.Parslow PA, Horne RSC, Cranage SM, Harding R, Adamson TM. Effects of maternal smoking on infant arousal responses to somatosensory and chemosensory stimuli. J Sleep Res. 2002;11:169–70. [Google Scholar]
- 15.Franco P, Chabanski S, Szliwowski H, Dramaix M, Kahn A. Influence of maternal smoking on autonomic nervous system in healthy infants. Pediatr Res. 2000;47:1–6. doi: 10.1203/00006450-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Kahn A, Groswasser J, Sottiaux M, et al. Prenatal exposure to cigarettes in infants with obstructive apneas. Pediatrics. 1994;93:778–83. [PubMed] [Google Scholar]
- 17.Longo LO. The biological effects of carbon monoxide on the pregnant woman, fetus, and newborn infant. Am J Obstet Gynecol. 1977;129:69–103. doi: 10.1016/0002-9378(77)90824-9. [DOI] [PubMed] [Google Scholar]
- 18.Fried PA, Watkinson B, Dillon RF, Dulberg CS. Neonatal neurological status in a low-risk population after prenatal exposure to cigarettes, marijuana, and alcohol. J Dev Behav Pediatr. 1987;8:318–26. [PubMed] [Google Scholar]
- 19.Ajarem JS, Ahmad M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav. 1998;59:313–8. doi: 10.1016/s0091-3057(97)00408-5. [DOI] [PubMed] [Google Scholar]
- 20.Pauly JR, Charriez CM, Guseva MV, Scheff SW. Nicotinic receptor modulation for neuroprotection and enhancement of functional recovery following brain injury or disease. Ann N Y Acad Sci. 2004;1035:316–34. doi: 10.1196/annals.1332.019. [DOI] [PubMed] [Google Scholar]
- 21.Franck MG, Srere H, Ledezma C, O'Hara B, Heller HC. Prenatal nicotine alters vigilance states and AchR gene expression in the neonatal rat: implication for SIDS. Am J Physiol Regulatory Integrative Comp Physiol. 2001;280:1134–40. doi: 10.1152/ajpregu.2001.280.4.R1134. [DOI] [PubMed] [Google Scholar]
- 22.George L, Granath F, Johansson AL, Cnattingius S. Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy. Acta Obstet Gynecol Scand. 2006;85:1331–7. doi: 10.1080/00016340600935433. [DOI] [PubMed] [Google Scholar]
- 23.McDonald SD, Perkins SL, Walker MC. Correlation between self-reported smoking status and serum cotinine during pregnancy. Addict Behav. 2005;30:853–7. doi: 10.1016/j.addbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Batstra L, Neeleman J, Hadders-Algra M. Can breast feeding modify the adverse effects of smoking during pregnancy on the child's cognitive development? J Epidemiol Community Health. 2003;57:403–4. doi: 10.1136/jech.57.6.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telliez F, Bach V, Leke A, Chardon K, Libert JP. Feeding behavior in neonates whose diet contained medium-chain triacylglycerols: short-term effects on thermoregulation and sleep. Am J Clin Nutr. 2002;76:1091–5. doi: 10.1093/ajcn/76.5.1091. [DOI] [PubMed] [Google Scholar]
- 26.Bach V, Telliez F, Libert JP. The interaction between sleep and thermoregulation in adults and neonates. Sleep Med Rev. 2002;6:481–92. doi: 10.1053/smrv.2001.0177. [DOI] [PubMed] [Google Scholar]
- 27.Curzi-Dascalova L, Mirmiran M, editors. Manual of methods for recording and analyzing sleep-wakefulness states in preterm and full-term infant. Paris: Editions INSERM; 1996. [Google Scholar]
- 28.Rediehs MH, Reis JS, Creason NS. Sleep in old age: focus on gender differences. Sleep. 1990;13:410–24. [PubMed] [Google Scholar]
- 29.Parmelee AH, Wenner WH, Schulz HR. Infant sleep patterns: from birth to 16 weeks of age. J Pediatr. 1964;65:576–82. doi: 10.1016/s0022-3476(64)80291-2. [DOI] [PubMed] [Google Scholar]
- 30.Winer G. Statistical principles in experiment design. New York: McGraw-Hill; 1971. [Google Scholar]
- 31.Telliez F, Bach V, Delanaud S, Leke A, Abdiche M, Chardon K. Influence of incubator humidity on sleep and behaviour of neonates kept at stable body temperature. Acta Paediatr. 2001;90:998–1003. doi: 10.1080/080352501316978066. [DOI] [PubMed] [Google Scholar]
- 32.CHIME Study Group. Hoppenbrouwers T, Hodgman JE, Rybine D, Fabrikant G, Corwin M, Crowell D, Weese-Mayer DE. Sleep architecture in term and preterm infants beyond the neonatal period: the influence of gestational age, steroids, and ventilatory support. Sleep. 2005;28:1428–36. doi: 10.1093/sleep/28.11.1428. [DOI] [PubMed] [Google Scholar]
- 33.Anders TF, Roffwarg HP. The effects of selective interruption and deprivation of sleep in the human newborn. Dev Psychobiol. 1973;6:79–91. doi: 10.1002/dev.420060110. [DOI] [PubMed] [Google Scholar]
- 34.Thomas DA, Poole K, McArdle EK, et al. The effect of sleep deprivation on sleep states, breathing events, peripheral chemoresponsiveness and arousal propensity in healthy 3 months old infants. Eur Respi J. 1996;9:932–8. doi: 10.1183/09031936.96.09050932. [DOI] [PubMed] [Google Scholar]
- 35.Lehtonen L, Martin RJ. Ontogeny of sleep and awake states in relation to breathing in preterm infants. Semin Neonatol. 2004;9:229–38. doi: 10.1016/j.siny.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. A prospective study of smoking during pregnancy and SIDS. Arch Dis Child. 2000;83:203–6. doi: 10.1136/adc.83.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horne RS, Franco P, Adamson TM, Groswasser J, Kahn A. Influences of maternal cigarette smoking on infant arousability. Early Hum Dev. 2004;79:49–58. doi: 10.1016/j.earlhumdev.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Fajardo B, Browning M, Fisher D, Paton J. Effect of nursery environment on state regulation in very-low-birthweight premature infants. Infant Behav Dev. 1990;13:287–303. [Google Scholar]
- 39.Anders TF, Keener MA. Developmental course of nighttime sleep-wake patterns in full-term and premature infants during the first year of life. Sleep. 1985;8:173–92. doi: 10.1093/sleep/8.3.173. [DOI] [PubMed] [Google Scholar]
- 40.Gaultier C. Apnea and sleep state in newborns and infants. Biol Neonate. 1994;65:231–4. doi: 10.1159/000244057. [DOI] [PubMed] [Google Scholar]
- 41.Harper RM, Kinney CH, Fleming PJ, Thach BT. Sleep influences on homeostatic functions: implications for sudden infant death syndrome. Resp Physiol. 2000;119:123–32. doi: 10.1016/s0034-5687(99)00107-3. [DOI] [PubMed] [Google Scholar]
- 42.Chardon K, Bach V, Telliez F, et al. Effect of caffeine on peripheral chemoreceptor activity in premature neonates: interaction with sleep stages. J Appl Physiol. 2004;96:2161–6. doi: 10.1152/japplphysiol.01160.2003. [DOI] [PubMed] [Google Scholar]
- 43.Wasserman RC, Kelleher KJ, Bocian A, et al. Identification of attentional and hyperactivity problems in primary care: a report from pediatric research in office settings and the ambulatory sentinel practice network. Pediatrics. 1999;103:E38. doi: 10.1542/peds.103.3.e38. [DOI] [PubMed] [Google Scholar]
- 44.Fried PA, O'Connell CM, Watkinson B. 60 and 72-month follow-up of children prenatally exposed to marijuana, cigarettes and alcohol: cognitive and language assessments. J Dev Behav Pediatr. 1992;13:383–91. [PubMed] [Google Scholar]
- 45.Borghese IF, Minard KL, Thoman EB. Sleep rhythmicity in premature infants: Implications for developmental status. Sleep. 1995;18:523–30. doi: 10.1093/sleep/18.7.523. [DOI] [PubMed] [Google Scholar]
- 46.Whitney MP, Thoman EB. Early patterns of premature infants are differentially related to later developmental disabilities. J Dev Behav Pediatr. 1993;14:71–80. [PubMed] [Google Scholar]