Abstract
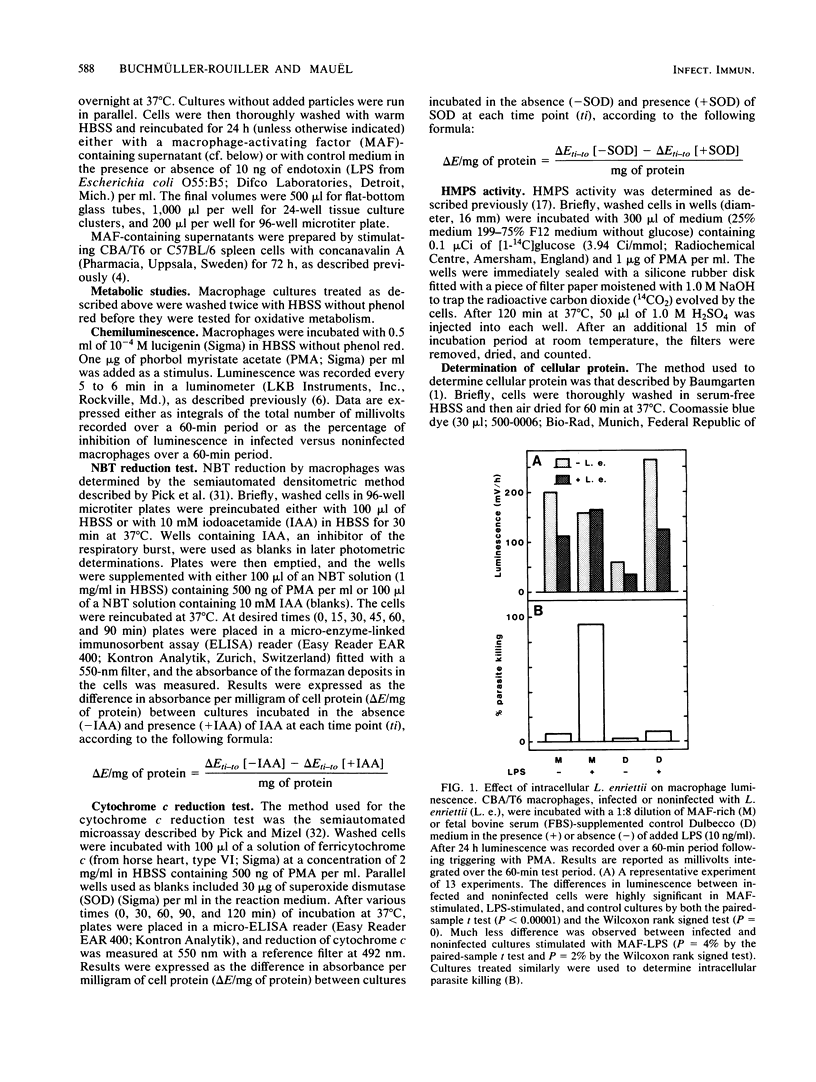
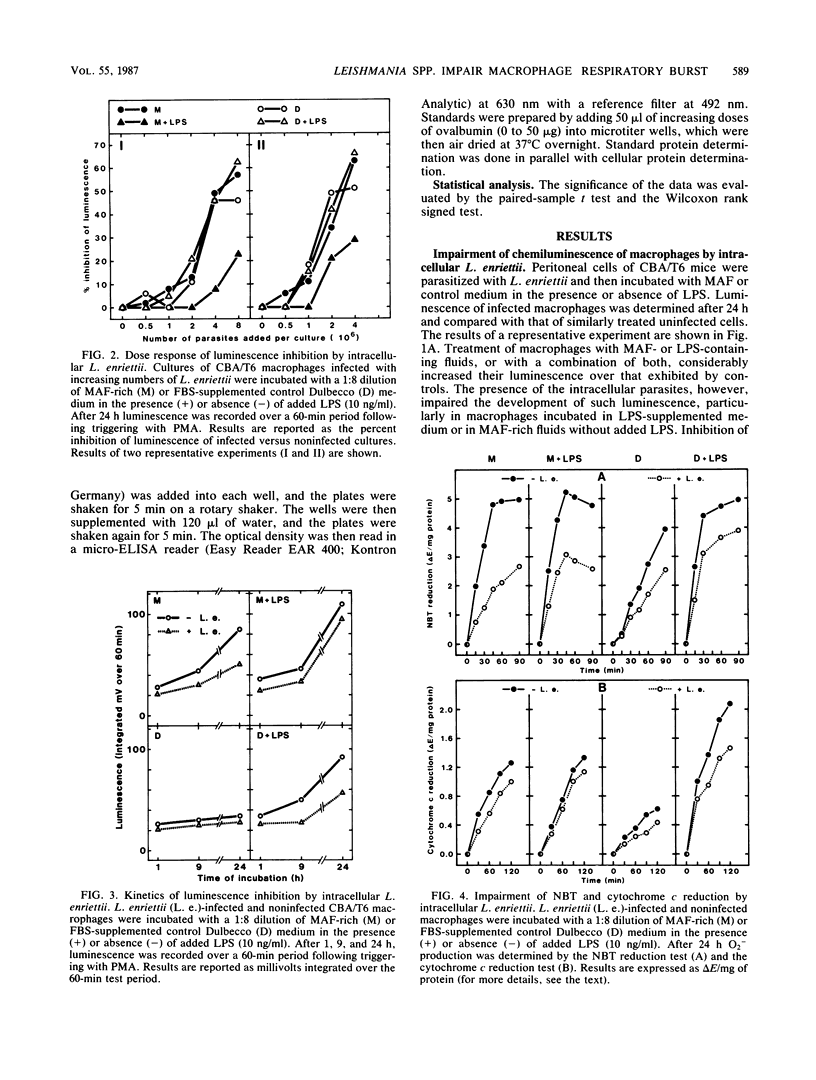
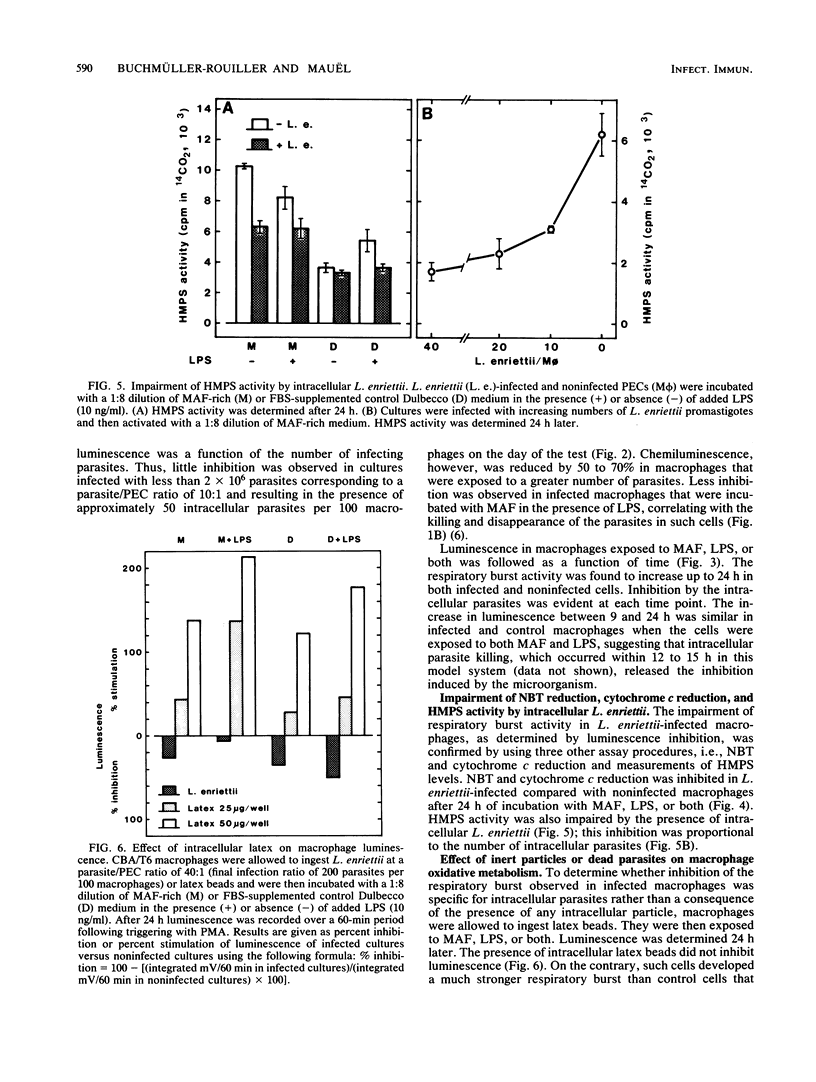
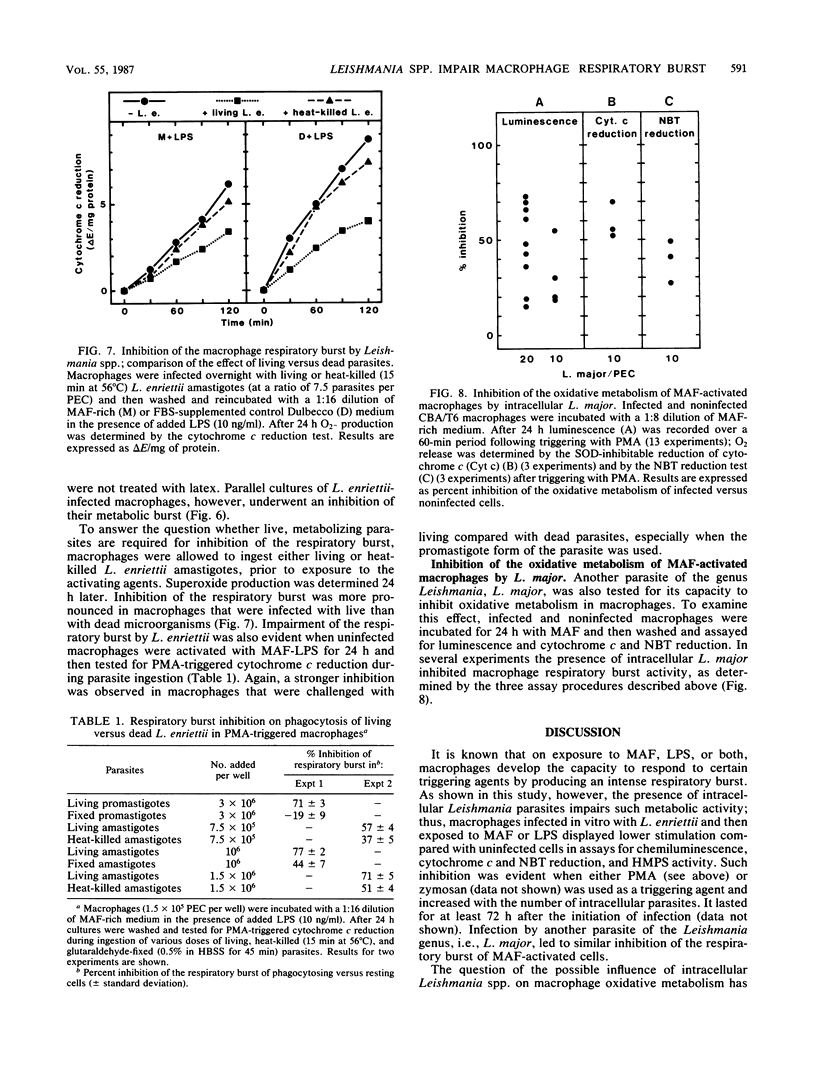
When stimulated in vitro with macrophage-activating factor or lipopolysaccharide, mouse peritoneal macrophages acquire the capacity to develop a strong respiratory burst when they are triggered by membrane-active agents. The presence of intracellular parasites of the genus Leishmania (L. enriettii, L. major) significantly inhibited such activity, as measured by chemiluminescence, reduction of cytochrome c and Nitro Blue Tetrazolium, and hexose monophosphate shunt levels. On the contrary, inert intracellular particles such as latex beads strongly increased the macrophage respiratory burst, suggesting that the Leishmania-linked inhibition resulted from a specific parasite effect. Impairment of macrophage oxidative metabolism by intracellular Leishmania spp. was a function of the number of infecting microorganisms and was more pronounced in macrophages infected with living than with dead parasites. Moreover, the metabolic inhibition was less apparent in L. enriettii-infected macrophages that were exposed to both macrophage-activating factor and lipopolysaccharide, i.e., conditions leading to complete parasite destruction. The mechanisms of respiratory burst inhibition by intracellular Leishmania spp. are unclear, but these observations suggest that such effects may contribute significantly to intracellular survival of the microorganisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgarten H. A simple microplate assay for the determination of cellular protein. J Immunol Methods. 1985 Sep 3;82(1):25–37. doi: 10.1016/0022-1759(85)90221-2. [DOI] [PubMed] [Google Scholar]
- Baxter M. A., Leslie R. G., Reeves W. G. The stimulation of superoxide anion production in guinea-pig peritoneal macrophages and neutrophils by phorbol myristate acetate, opsonized zymosan and IgG2-containing soluble immune complexes. Immunology. 1983 Apr;48(4):657–665. [PMC free article] [PubMed] [Google Scholar]
- Berens R. L., Marr J. J. An easily prepared defined medium for cultivation of Leishmania donovani promastigotes. J Parasitol. 1978 Feb;64(1):160–160. [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Correlation between enhanced oxidative metabolism and leishmanicidal activity in activated macrophages from healer and nonhealer mouse strains. J Immunol. 1986 May 15;136(10):3884–3890. [PubMed] [Google Scholar]
- Buchmüller Y., Mauel J. Studies on the mechanisms of macrophage activation. II. Parasite destruction in macrophages activated by supernates from concanavalin A-stimulated lymphocytes. J Exp Med. 1979 Aug 1;150(2):359–370. doi: 10.1084/jem.150.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmüller Y., Mauel J. Studies on the mechanisms of macrophage activation: possible involvement of oxygen metabolites in killing of Leishmania enrietti by activated mouse macrophages. J Reticuloendothel Soc. 1981 Mar;29(3):181–192. [PubMed] [Google Scholar]
- Channon J. Y., Blackwell J. M. A study of the sensitivity of Leishmania donovani promastigotes and amastigotes to hydrogen peroxide. II. Possible mechanisms involved in protective H2O2 scavenging. Parasitology. 1985 Oct;91(Pt 2):207–217. doi: 10.1017/s0031182000057310. [DOI] [PubMed] [Google Scholar]
- Channon J. Y., Roberts M. B., Blackwell J. M. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology. 1984 Oct;53(2):345–355. [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Long G. D., Shirley P. S., Bass D. A., Thomas M. J., Henderson F. W., Cohen M. S. Mechanism of the luminol-dependent chemiluminescence of human neutrophils. J Immunol. 1982 Oct;129(4):1589–1593. [PubMed] [Google Scholar]
- DiStefano J. F., Beck G., Zucker S. Mechanism of BCG-activated macrophage-induced tumor cell cytotoxicity: evidence for both oxygen-dependent and independent mechanisms. Int Arch Allergy Appl Immunol. 1983 Mar;70(3):252–260. doi: 10.1159/000233332. [DOI] [PubMed] [Google Scholar]
- Eilam Y., El-On J., Spira D. T. Leishmania major: excreted factor, calcium ions, and the survival of amastigotes. Exp Parasitol. 1985 Apr;59(2):161–168. doi: 10.1016/0014-4894(85)90068-2. [DOI] [PubMed] [Google Scholar]
- Gottlieb M., Dwyer D. M. Leishmania donovani: surface membrane acid phosphatase activity of promastigotes. Exp Parasitol. 1981 Aug;52(1):117–128. doi: 10.1016/0014-4894(81)90067-9. [DOI] [PubMed] [Google Scholar]
- Haidaris C. G., Bonventre P. F. A role for oxygen-dependent mechanisms in killing of Leishmania donovani tissue forms by activated macrophages. J Immunol. 1982 Aug;129(2):850–855. [PubMed] [Google Scholar]
- Locksley R. M., Klebanoff S. J. Oxygen-dependent microbicidal systems of phagocytes and host defense against intracellular protozoa. J Cell Biochem. 1983;22(3):173–185. doi: 10.1002/jcb.240220306. [DOI] [PubMed] [Google Scholar]
- Mauel J., Behin R., Biroum-Noerjasin, Rowe D. S. Mechanisms of protective immunity in experimental cutaneous leishmaniasis of the guinea-pig. I. Lack of effects of immune lymphocytes and of activated macrophages. Clin Exp Immunol. 1975 May;20(2):339–350. [PMC free article] [PubMed] [Google Scholar]
- Mauel J., Buchmüller Y., Behin R. Studies on the mechanisms of macrophage activation. I. Destruction of intracellular Leishmania enriettii in macrophages activated by cocultivation with stimulated lymphocytes. J Exp Med. 1978 Aug 1;148(2):393–407. doi: 10.1084/jem.148.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauel J., Schnyder J., Baggiolini M. Intracellular parasite killing induced by electron carriers. II. Correlation between parasite killing and the induction of oxidative events in macrophages. Mol Biochem Parasitol. 1984 Sep;13(1):97–110. doi: 10.1016/0166-6851(84)90104-x. [DOI] [PubMed] [Google Scholar]
- Meshnick S. R., Eaton J. W. Leishmanial superoxide dismutase: a possible target for chemotherapy. Biochem Biophys Res Commun. 1981 Oct 15;102(3):970–976. doi: 10.1016/0006-291x(81)91633-8. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol. 1982 Jul;129(1):351–357. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Macrophage activation: enhanced oxidative and antiprotozoal activity. Contemp Top Immunobiol. 1984;13:97–115. doi: 10.1007/978-1-4757-1445-6_5. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Pretreatment with phorbol myristate acetate inhibits macrophage activity against intracellular protozoa. J Reticuloendothel Soc. 1982 Jun;31(6):479–487. [PubMed] [Google Scholar]
- Murray H. W. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med. 1981 May 1;153(5):1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A. A., Honigberg B. M. Leishmania mexicana pifanoi: in vivo and in vitro interactions between amastigotes and macrophages. Z Parasitenkd. 1985;71(1):3–13. doi: 10.1007/BF00932913. [DOI] [PubMed] [Google Scholar]
- Passwell J., Shor R., Keren G., Messer G., El-On J. Comparison of the effect of various stimuli on the leishmaniacidal capacity of human monocytes in vitro. Clin Exp Immunol. 1984 Jun;56(3):553–558. [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Harcus J. L., Symes P. H., Romito R., Donowitz G. R. Failure of the phagocytic oxidative response to protect human monocyte-derived macrophages from infection by Leishmania donovani. J Immunol. 1982 Sep;129(3):1282–1286. [PubMed] [Google Scholar]
- Philippeaux M. M., Mauel J. Extracellular cytolysis by activated macrophages: studies with macrophages on permeable membranes. Immunobiology. 1984 Oct;167(4):301–317. doi: 10.1016/S0171-2985(84)80002-9. [DOI] [PubMed] [Google Scholar]
- Pick E., Charon J., Mizel D. A rapid densitometric microassay for nitroblue tetrazolium reduction and application of the microassay to macrophages. J Reticuloendothel Soc. 1981 Dec;30(6):581–593. [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Remaley A. T., Das S., Campbell P. I., LaRocca G. M., Pope M. T., Glew R. H. Characterization of Leishmania donovani acid phosphatases. J Biol Chem. 1985 Jan 25;260(2):880–886. [PubMed] [Google Scholar]
- Remaley A. T., Glew R. H., Kuhns D. B., Basford R. E., Waggoner A. S., Ernst L. A., Pope M. Leishmania donovani: surface membrane acid phosphatase blocks neutrophil oxidative metabolite production. Exp Parasitol. 1985 Dec;60(3):331–341. doi: 10.1016/0014-4894(85)90039-6. [DOI] [PubMed] [Google Scholar]
- Scott P., James S., Sher A. The respiratory burst is not required for killing of intracellular and extracellular parasites by a lymphokine-activated macrophage cell line. Eur J Immunol. 1985 Jun;15(6):553–558. doi: 10.1002/eji.1830150605. [DOI] [PubMed] [Google Scholar]


