Abstract
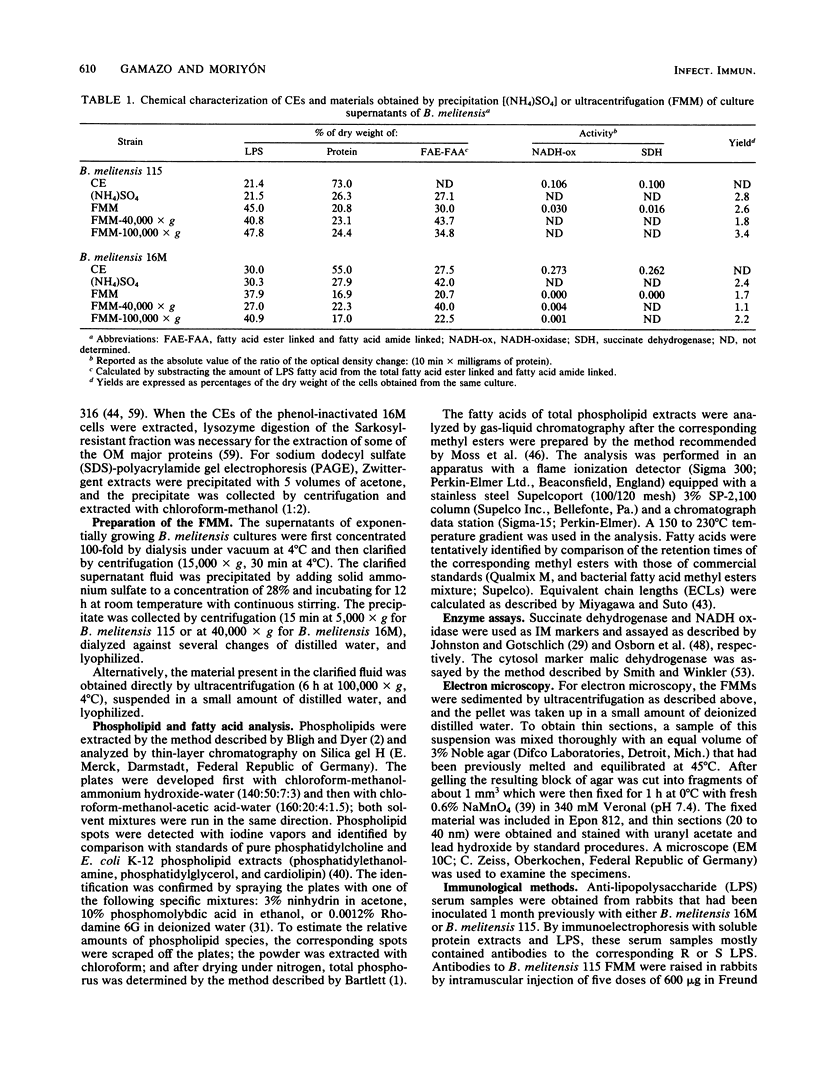
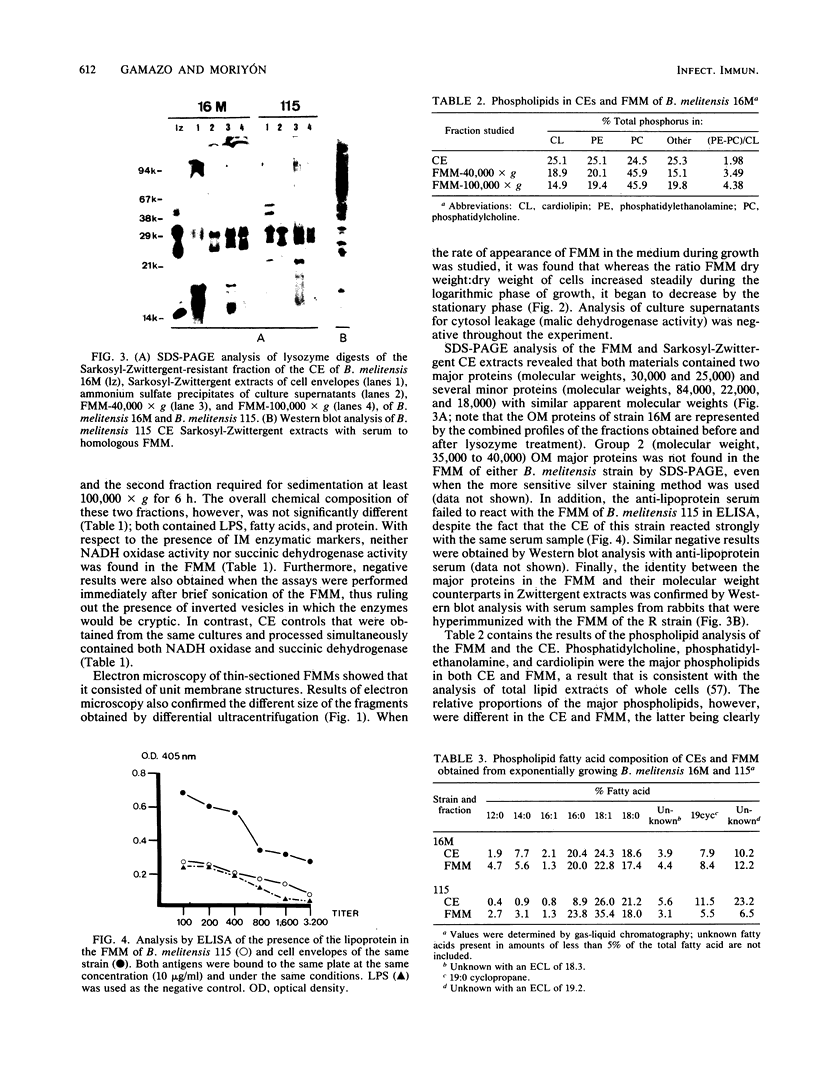
Rough and smooth strains of Brucella melitensis released a membranous material that was devoid of detectable NADH oxidase and succinic dehydrogenase activity (cytoplasmic membrane markers) but that contained lipopolysaccharide, proteins, and phospholipids. This material was composed of two fractions that had similar chemical compositions but that were of different sizes which were separated by differential ultracentrifugation. Electron microscopy showed that both fractions are made of unit membrane structures. The membrane fragments were released during the exponential phase of growth, and no leakage of malic dehydrogenase activity (cytosol marker) was detected. Thus, the fragments were unlikely a result of cell lysis. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis showed that, although group 2 Brucella outer membrane proteins and lipoprotein were not detected, the proteins in the membranous material were outer membrane proteins. Gas-liquid chromatography analysis showed a similar fatty acid profile for the cell envelope and the outer membrane fragments of the smooth strain B. melitensis 16M. In contrast, the outer membrane fragments from the rough 115 strain were enriched in palmitic and stearic acids. With respect to the unfractionated cell envelope, outer membrane fragments were enriched in phosphatidylcholine, a phospholipid that is unusual in bacterial membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. N., Das J. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J Gen Microbiol. 1967 Oct;49(1):1–11. doi: 10.1099/00221287-49-1-1. [DOI] [PubMed] [Google Scholar]
- Chopra I., Shales S. W. Comparison of the polypeptide composition of Escherichia coli outer membranes prepared by two methods. J Bacteriol. 1980 Oct;144(1):425–427. doi: 10.1128/jb.144.1.425-427.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Dees S., Thanabalasundrum S., Moss C. W., Hollis D. G., Weaver R. E. Cellular fatty acid composition of group IVe, a nonsaccharolytic organism from clinical sources. J Clin Microbiol. 1980 Jun;11(6):664–668. doi: 10.1128/jcm.11.6.664-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Wilson J. B. Antigenic relationship of Brucella ovis and Brucella melitensis. J Bacteriol. 1967 Apr;93(4):1262–1268. doi: 10.1128/jb.93.4.1262-1268.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich D. L., Cota-Robles E. H. Heterogeneity in lipid composition of the outer membrane and cytoplasmic membrane and cytoplasmic membrane of Pseudomonas BAL-31. J Bacteriol. 1974 Sep;119(3):1006–1018. doi: 10.1128/jb.119.3.1006-1018.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G. Etude ultrastructurale des bactéries de colonies lisses (S) et rugueuses (R) du genre "Brucella". Ann Inst Pasteur (Paris) 1972 Aug;123(2):171–193. [PubMed] [Google Scholar]
- Dubray G. Localisation cellulaire des polyosides des bactéries des genres Brucella et Escherichia en phase lisse (S) ou rugueuse (R) Ann Microbiol (Paris) 1976 Aug-Sep;127B(2):133–149. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fung J., MacAlister T. J., Rothfield L. I. Role of murein lipoprotein in morphogenesis of the bacterial division septum: phenotypic similarity of lkyD and lpo mutants. J Bacteriol. 1978 Mar;133(3):1467–1471. doi: 10.1128/jb.133.3.1467-1471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gankema H., Wensink J., Guinée P. A., Jansen W. H., Witholt B. Some characteristics of the outer membrane material released by growing enterotoxigenic Escherichia coli. Infect Immun. 1980 Aug;29(2):704–713. doi: 10.1128/iai.29.2.704-713.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular organization of the outer membrane of Salmonella typhimurium. Eur J Biochem. 1979 Feb 1;93(3):609–620. doi: 10.1111/j.1432-1033.1979.tb12861.x. [DOI] [PubMed] [Google Scholar]
- Goldfine H. Bacterial membranes and lipid packing theory. J Lipid Res. 1984 Dec 15;25(13):1501–1507. [PubMed] [Google Scholar]
- Gómez-Miguel M. J., Moriyón I. Demonstration of a peptidoglycan-linked lipoprotein and characterization of its trypsin fragment in the outer membrane of Brucella spp. Infect Immun. 1986 Sep;53(3):678–684. doi: 10.1128/iai.53.3.678-684.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Miguel M. J., Moriyón I., López J. Brucella outer membrane lipoprotein shares antigenic determinants with Escherichia coli Braun lipoprotein and is exposed on the cell surface. Infect Immun. 1987 Jan;55(1):258–262. doi: 10.1128/iai.55.1.258-262.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., van der Laan J. W., de Leij L., Witholt B. Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta. 1976 Dec 14;455(3):889–899. doi: 10.1016/0005-2736(76)90058-4. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Tanner A. C., Socransky S. S. Morphology and ultrastructure of oral strains of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. Infect Immun. 1980 Nov;30(2):588–600. doi: 10.1128/iai.30.2.588-600.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishinaga M., Kanamoto R., Kito M. Distribution of phospholipid molecular species in outer and cytoplasmic membrane of Escherichia coli. J Biochem. 1979 Jul;86(1):161–165. [PubMed] [Google Scholar]
- Jantzen E., Knudsen E., Winsnes R. Fatty acid analysis for differentiation or Bordetella and Brucella species. Acta Pathol Microbiol Immunol Scand B. 1982 Oct;90(5):353–359. doi: 10.1111/j.1699-0463.1982.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. M., Diaz R., Berman D. T. Endotoxic activity of rough organisms of Brucella species. Infect Immun. 1976 Jun;13(6):1638–1641. doi: 10.1128/iai.13.6.1638-1641.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Cullen J., Work E. An extracellular lipopolysaccharide-phospholipid-protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1967 Apr;103(1):192–201. doi: 10.1042/bj1030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G. A rapid slide-agglutination method for typing pneumococci by means of specific antibody adsorbed to protein A-containing staphylococci. J Med Microbiol. 1973 May;6(2):187–190. doi: 10.1099/00222615-6-2-187. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Permanganate; a new fixative for electron microscopy. J Biophys Biochem Cytol. 1956 Nov 25;2(6):799–802. doi: 10.1083/jcb.2.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong D., Diaz R., Milner K., Rudbach J., Wilson J. B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970 Feb;1(2):174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Kilner J. Release of a special fraction of the outer membrane from both growing and phage T4-infected Escherichia coli B. Biochim Biophys Acta. 1978 Dec 4;514(1):117–127. doi: 10.1016/0005-2736(78)90081-0. [DOI] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Outer membrane characteristics of Campylobacter jejuni. Infect Immun. 1982 Dec;38(3):898–906. doi: 10.1128/iai.38.3.898-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., Peters R. Distribution of lipids in cytoplasmic and outer membranes of Escherichia coli K12. Biochim Biophys Acta. 1976 Jul 20;441(1):38–47. doi: 10.1016/0005-2760(76)90279-4. [DOI] [PubMed] [Google Scholar]
- MacIntyre S., Trust T. J., Buckley J. T. Identification and characterization of outer membrane fragments released by Aeromonas sp. Can J Biochem. 1980 Oct;58(10):1018–1025. doi: 10.1139/o80-138. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Effects of nonionic, ionic, and dipolar ionic detergents and EDTA on the Brucella cell envelope. J Bacteriol. 1982 Nov;152(2):822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Isolation, purification, and partial characterization of Brucella abortus matrix protein. Infect Immun. 1983 Jan;39(1):394–402. doi: 10.1128/iai.39.1.394-402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Lambert M. A., Merwin W. H. Comparison of rapid methods for analysis of bacterial fatty acids. Appl Microbiol. 1974 Jul;28(1):80–85. doi: 10.1128/am.28.1.80-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mug-Opstelten D., Witholt B. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim Biophys Acta. 1978 Apr 4;508(2):287–295. doi: 10.1016/0005-2736(78)90331-0. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Santos J. M., Verstreate D. R., Perera V. Y., Winter A. J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984 Oct;46(1):188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Osborn M. J., Koppel D. E. Lateral mobility in reconstituted membranes--comparisons with diffusion in polymers. Nature. 1980 Jan 24;283(5745):346–350. doi: 10.1038/283346a0. [DOI] [PubMed] [Google Scholar]
- Smith D. K., Winkler H. H. Separation of inner and outer membranes of Rickettsia prowazeki and characterization of their polypeptide compositions. J Bacteriol. 1979 Feb;137(2):963–971. doi: 10.1128/jb.137.2.963-971.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele O. W., Asselineau J., Lacave C. On the fatty acids of Brucella abortus and Brucella melitensis. Eur J Biochem. 1969 Jan;7(3):393–396. doi: 10.1111/j.1432-1033.1969.tb19621.x. [DOI] [PubMed] [Google Scholar]
- Thiele O. W., Schwinn G. The free lipids of Brucella melitensis and Bordetella pertussis. Eur J Biochem. 1973 Apr;34(2):333–344. doi: 10.1111/j.1432-1033.1973.tb02764.x. [DOI] [PubMed] [Google Scholar]
- Thilo L., Overath P. Randomization of membrane lipids in relation to transport system assembly in Escherichia coli. Biochemistry. 1976 Jan 27;15(2):328–334. doi: 10.1021/bi00647a014. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weigand R. A., Vinci K. D., Rothfield L. I. Morphogenesis of the bacterial division septum: a new class of septation-defective mutants. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1882–1886. doi: 10.1073/pnas.73.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink J., Witholt B. Outer-membrane vesicles released by normally growing Escherichia coli contain very little lipoprotein. Eur J Biochem. 1981 May 15;116(2):331–335. doi: 10.1111/j.1432-1033.1981.tb05338.x. [DOI] [PubMed] [Google Scholar]
- Woo D. D., Holt S. C., Leadbetter E. R. Ultrastructure of Bacteroides species: Bacteroides asaccharolyticus, Bacteroides fragilis, Bacteroides melaninogenicus subspecies melaninogenicus, and B. melaninogenicus subspecies intermedius. J Infect Dis. 1979 May;139(5):534–546. doi: 10.1093/infdis/139.5.534. [DOI] [PubMed] [Google Scholar]
- Yem D. W., Wu H. C. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol. 1978 Mar;133(3):1419–1426. doi: 10.1128/jb.133.3.1419-1426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leij L., Kingma J., Witholt B. Nature of the regions involved in the insertion of newly synthesized protein into the outer membrane of Escherichia coli. Biochim Biophys Acta. 1979 May 17;553(2):224–234. doi: 10.1016/0005-2736(79)90227-x. [DOI] [PubMed] [Google Scholar]




