Abstract
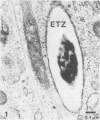
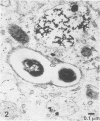
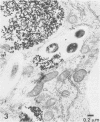
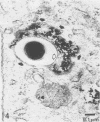
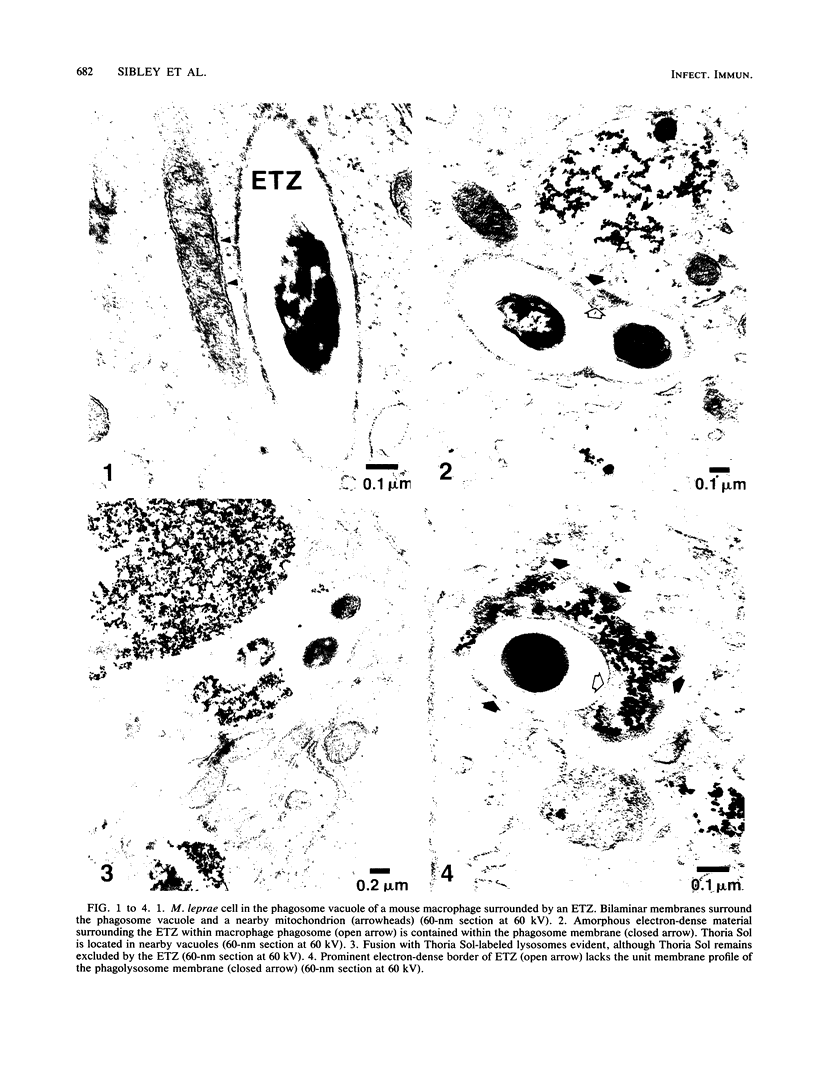
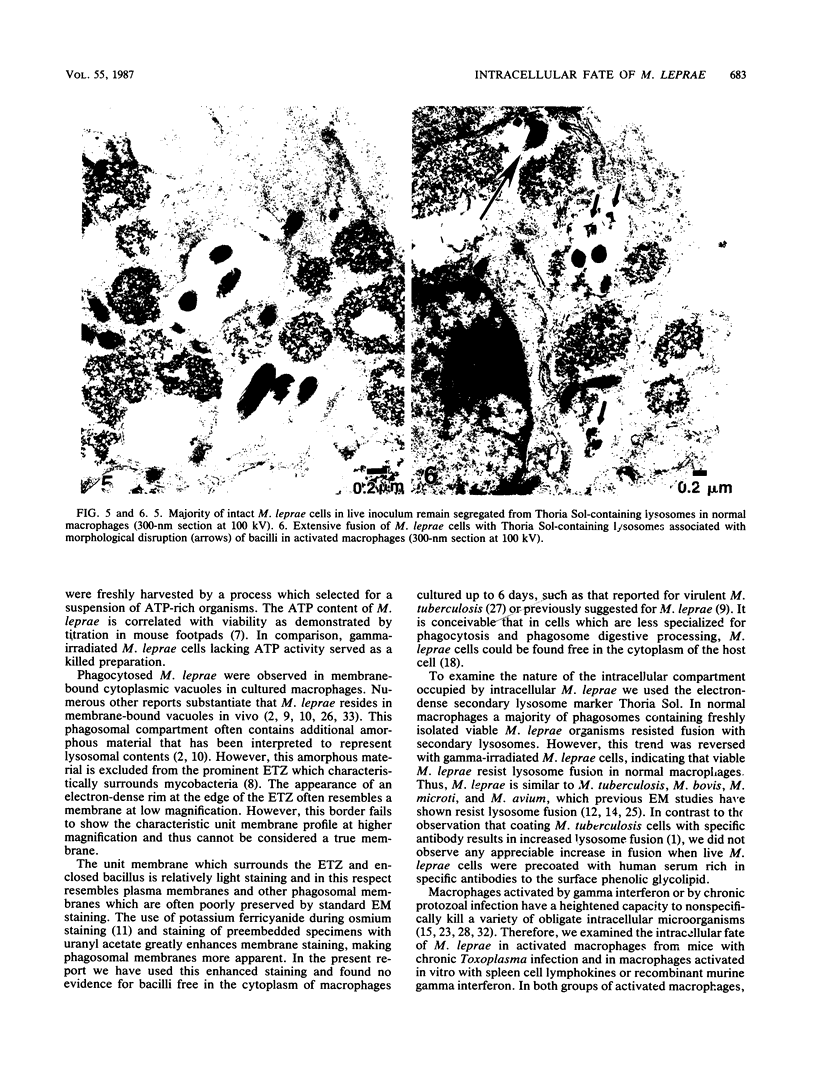
Mycobacterium leprae replicates within mononuclear phagocytes, reaching enormous numbers in the macrophage-rich granulomas of lepromatous leprosy. To examine the capability of macrophages to digest M. leprae, we studied the intracellular fate of M. leprae organisms in normal and activated mouse macrophages by using the electron-dense secondary lysosome tracer Thoria Sol. Intracellular M. leprae organisms, surrounded by a characteristic electron-transparent zone, were contained within phagosomal vacuoles of macrophages cultured in vitro for 1 to 6 days. In normal macrophages, a majority of phagosomes containing freshly isolated live M. leprae cells resisted fusion with Thoria Sol-labeled lysosomes. The extent of fusion was not significantly affected by pretreatment of M. leprae with human patient serum high in specific immunoglobulin G and M antibodies. In contrast, a majority of phagosomes containing gamma-irradiated M. leprae cells underwent lysosome fusion in normal macrophages. In addition, increased phagolysosome fusion was observed with live M. leprae-containing phagosomes in macrophages activated with gamma interferon. Increased fusion was associated with an increase in the number of fragmented and damaged bacilli, suggesting that increased digestion followed fusion. This study indicates that activated macrophages may have an increased capacity for clearance of normally resistant M. leprae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandi S. M., Job C. K. The early cellular response to M. leprae. An ultrastructural study. Lepr India. 1978 Jul;50(3):345–357. [PubMed] [Google Scholar]
- Chehl S., Ruby J., Job C. K., Hastings R. C. The growth of Mycobacterium leprae in nude mice. Lepr Rev. 1983 Dec;54(4):283–304. doi: 10.5935/0305-7518.19830035. [DOI] [PubMed] [Google Scholar]
- Convit J., Aranzazu N., Pinardi M., Ulrich M. Immunological changes observed in indeterminate and lepromatous leprosy patients and Mitsuda-negative contacts after the inoculation of a mixture of Mycobacterium leprae and BCG. Clin Exp Immunol. 1979 May;36(2):214–220. [PMC free article] [PubMed] [Google Scholar]
- Convit J., Pinardi M. E., Rodríguez Ochoa G., Ulrich M., Avila J. L., Goihman M. Elimination of Mycobacterium leprae subsequent to local in vivo activation of macrophages in lepromatous leprosy by other mycobacteria. Clin Exp Immunol. 1974 Jun;17(2):261–265. [PMC free article] [PubMed] [Google Scholar]
- Dhople A. M. Adenosine triphosphate content of Mycobacterium leprae from leprosy patients. Int J Lepr Other Mycobact Dis. 1984 Jun;52(2):183–188. [PubMed] [Google Scholar]
- Draper P., Rees R. J. Electron-transparent zone of mycobacteria may be a defence mechanism. Nature. 1970 Nov 28;228(5274):860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Levy L. Ultrastructural changes in cells of the mouse footpad infected with Mycobacterium leprae. Infect Immun. 1972 Feb;5(2):238–247. doi: 10.1128/iai.5.2.238-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Newton H. E., Levy L. Early response of mouse foot pads to Mycobacterium laprae. Infect Immun. 1973 Jan;7(1):76–85. doi: 10.1128/iai.7.1.76-85.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes M. S., Plantholt B. A., Sperelakis N. Cytochemical staining procedures selective for sarcotubular systems of muscle: modifications and applications. J Ultrastruct Res. 1977 Sep;60(3):306–327. doi: 10.1016/s0022-5320(77)80016-6. [DOI] [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Resistance to murine tumors conferred by chronic infection with intracellular protozoa, Toxoplasma gondii and Besnoitia jellisoni. J Infect Dis. 1971 Dec;124(6):587–592. doi: 10.1093/infdis/124.6.587. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Levis W. R., Cohn Z. A. Defective production of monocyte-activating cytokines in lepromatous leprosy. J Exp Med. 1984 Mar 1;159(3):666–678. doi: 10.1084/jem.159.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job C. K., Chehl S., Hastings R. C., Ruby J. R. Invasion of liver parenchymal cells by Mycobacterium leprae in an experimentally infected nude mouse. An electron microscopic study. Am J Trop Med Hyg. 1983 Sep;32(5):1088–1095. doi: 10.4269/ajtmh.1983.32.1088. [DOI] [PubMed] [Google Scholar]
- Job C. K. Lysosomal activity of macrophages in leprosy. Arch Pathol. 1970 Dec;90(6):547–552. [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Cohn Z. A. The immunobiology of leprosy. Int Rev Exp Pathol. 1986;28:45–78. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Humphres R. C., Henika P. C. Effects of Propionibacterium acnes treatment on the course of Mycobacterium leprae infection in mice. Infect Immun. 1982 Jul;37(1):183–188. doi: 10.1128/iai.37.1.183-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. The role of activated macrophages in specific and nonspecific cytostasis of tumor cells. J Immunol. 1974 Aug;113(2):507–516. [PubMed] [Google Scholar]
- Kvach J. T., Neubert T. A., Palomino J. C., Heine H. S. Adenosine triphosphate content of Mycobacterium leprae isolated from armadillo tissue by Percoll buoyant density centrifugation. Int J Lepr Other Mycobact Dis. 1986 Mar;54(1):1–10. [PubMed] [Google Scholar]
- Lowrie D. B., Aber V. R., Jackett P. S. Phagosome-lysosome fusion and cyclic adenosine 3':5'-monophosphate in macrophages infected with Mycobacterium microti, Mycobacterium bovis BCG or Mycobacterium lepraemurium. J Gen Microbiol. 1979 Feb;110(2):431–441. doi: 10.1099/00221287-110-2-431. [DOI] [PubMed] [Google Scholar]
- McKeever P. E., Walsh G. P., Storrs E. E., Balentine J. D. Electron microscopy of peroxidase and acid phosphatase in leprous and uninfected armadillo macrophages: a macrophage subpopulation contains peroxisomes and lacks bacilli. Am J Trop Med Hyg. 1978 Sep;27(5):1019–1029. doi: 10.4269/ajtmh.1978.27.1019. [DOI] [PubMed] [Google Scholar]
- Myrvik Q. N., Leake E. S., Wright M. J. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am Rev Respir Dis. 1984 Feb;129(2):322–328. [PubMed] [Google Scholar]
- Nathan C. F., Kaplan G., Levis W. R., Nusrat A., Witmer M. D., Sherwin S. A., Job C. K., Horowitz C. R., Steinman R. M., Cohn Z. A. Local and systemic effects of intradermal recombinant interferon-gamma in patients with lepromatous leprosy. N Engl J Med. 1986 Jul 3;315(1):6–15. doi: 10.1056/NEJM198607033150102. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51(5):451–465. [PMC free article] [PubMed] [Google Scholar]
- Ridley M. J. The mononuclear cell series in leprosy: an ultrastructural report. Lepr Rev. 1981 Mar;52(1):35–50. doi: 10.5935/0305-7518.19810006. [DOI] [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- Saito H., Tomioka H., Sato K., Watanabe T. Abilities of human oligodendroglial cells and mouse Schwann cells to phagocytose Mycobacterium leprae and other mycobacteria. Infect Immun. 1986 Jan;51(1):157–162. doi: 10.1128/iai.51.1.157-162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L., Weidner E. Lymphokine activation of J774G8 cells and mouse peritoneal macrophages challenged with Toxoplasma gondii. Infect Immun. 1985 Sep;49(3):760–764. doi: 10.1128/iai.49.3.760-764.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Crowle A. J. Lymphokine-induced mycobacteriostatic activity in mouse pleural macrophages. Infect Immun. 1982 Aug;37(2):786–793. doi: 10.1128/iai.37.2.786-793.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]