Abstract
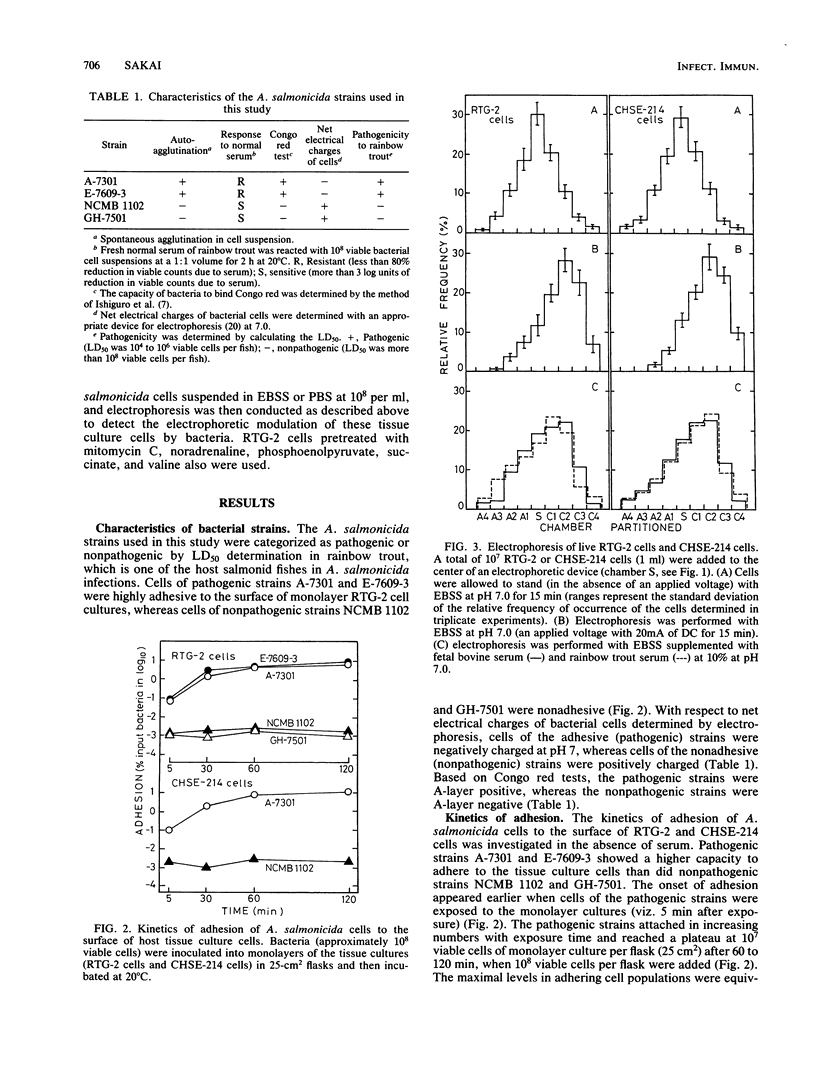
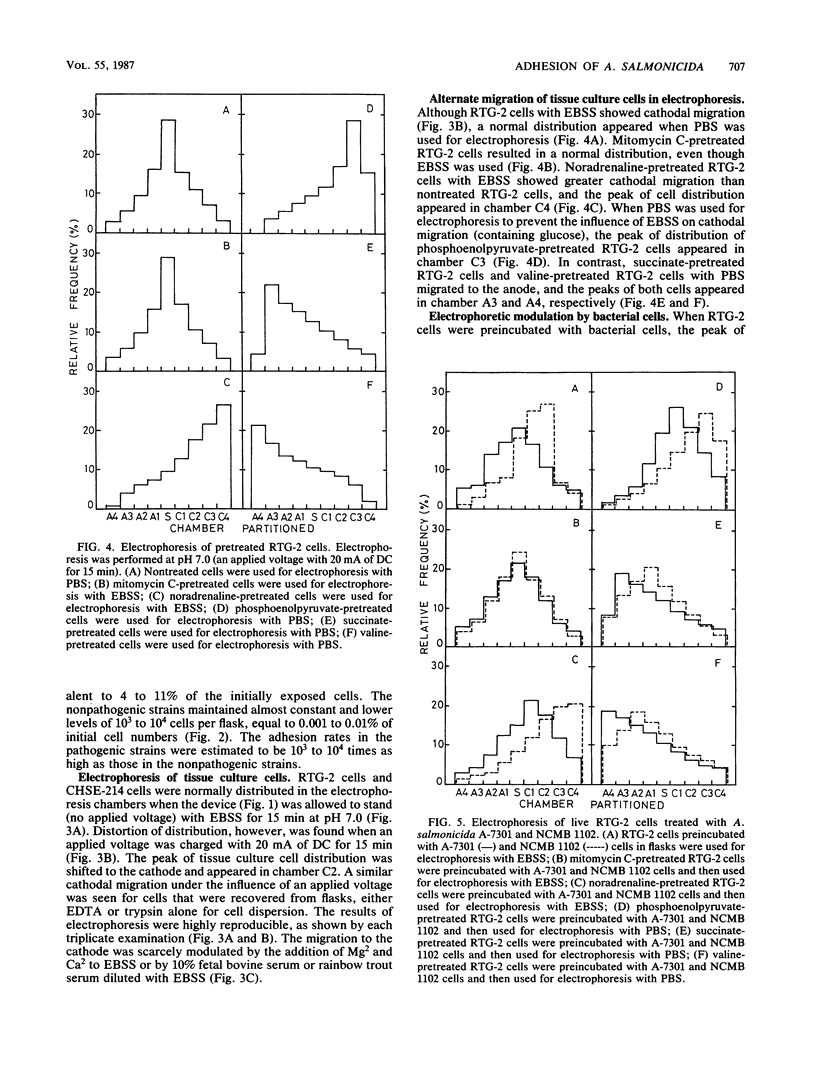
The adhesion of Aeromonas salmonicida, the pathogenic bacterium of fish furunculosis in salmon and trout, to the surface of host tissue cells was investigated with two fish tissue culture cell lines (RTG-2 cells from rainbow trout, Salmo gairdneri, and CHSE-214 cells from chinook salmon, Oncorhynchus tshawytscha) and four A. salmonicida strains. Bacterial cells of pathogenic strains were highly adhesive to RTG-2 and CHSE-214 cells and were negatively charged in the net electrostatic charges, as determined by electrophoresis on filter paper strips at pH 7, whereas bacterial cells of nonpathogenic strains were nonadhesive and positively charged. The electrophoresis of RTG-2 and CHSE-214 cells with balanced salt solution (BSS), phosphate-buffered saline, or fish serum diluted with BSS (pH 7) was carried out with an appropriate electrophoretic apparatus that was devised for this study. After electrophoresis with 20 mA of direct current for 15 min at pH 7, the electrophoretic dispositions of these tissue culture cells were determined by the mode of frequency of occurrence of these cells in the partitioned chambers of the device. RTG-2 and CHSE-214 cells with BSS and fish serum were attracted from the central chamber (to which each cell sample was added) to the cathode chambers, but no attraction was detected when these cells were used with phosphate-buffered saline. Noradrenaline- and phosphoenolpyruvate-pretreated RTG-2 cells migrated more to the cathode chambers, whereas succinate- and valine-pretreated RTG-2 cells moved to the anode chambers. These movements to the cathode and anode were alleviated by the use of RTG-2 cells preincubated with pathogenic and nonpathogenic bacterial cells, respectively. The adhesion of the pathogenic bacteria to RTG-2 cells was enhanced by the use of RTG-2 cells pretreated with noradrenaline and phosphoenolpyruvate, whereas the nonpathogenic bacteria were adherent to RTG-2 cells pretreated with succinate and valine. These findings indicate that the adhesion of A. salmonicida strains to host tissue cells is closely associated with mutually converse net electrostatic charges.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evenberg D., Lugtenberg B. Cell surface of the fish pathogenic bacterium Aeromonas salmonicida. II. Purification and characterization of a major cell envelope protein related to autoagglutination, adhesion and virulence. Biochim Biophys Acta. 1982 Jan 22;684(2):249–254. doi: 10.1016/0005-2736(82)90013-x. [DOI] [PubMed] [Google Scholar]
- Evenberg D., Van Boxtel R., Lugtenberg B., Schurer F., Blommaert J., Bootsma R. Cell surface of the fish pathogenic bacterium Aeromonas salmonicida. I. Relationship between autoagglutination and the presence of a major cell envelope protein. Biochim Biophys Acta. 1982 Jan 22;684(2):241–248. doi: 10.1016/0005-2736(82)90012-8. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Ainsworth T., Trust T. J., Kay W. W. Congo red agar, a differential medium for Aeromonas salmonicida, detects the presence of the cell surface protein array involved in virulence. J Bacteriol. 1985 Dec;164(3):1233–1237. doi: 10.1128/jb.164.3.1233-1237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Kay W. W., Ainsworth T., Chamberlain J. B., Austen R. A., Buckley J. T., Trust T. J. Loss of virulence during culture of Aeromonas salmonicida at high temperature. J Bacteriol. 1981 Oct;148(1):333–340. doi: 10.1128/jb.148.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Buckley J. T., Ishiguro E. E., Phipps B. M., Monette J. P., Trust T. J. Purification and disposition of a surface protein associated with virulence of Aeromonas salmonicida. J Bacteriol. 1981 Sep;147(3):1077–1084. doi: 10.1128/jb.147.3.1077-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn C. B., Ishiguro E. E., Kay W. W., Trust T. J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect Immun. 1982 Jun;36(3):1069–1075. doi: 10.1128/iai.36.3.1069-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D. K. Loss of virulence in a protease-deficient mutant of Aeromonas salmonicida. Infect Immun. 1985 Apr;48(1):146–152. doi: 10.1128/iai.48.1.146-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D. K. Significance of Extracellular Protease for Growth of a Heterotrophic Bacterium, Aeromonas salmonicida. Appl Environ Microbiol. 1985 Oct;50(4):1031–1037. doi: 10.1128/aem.50.4.1031-1037.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


