Abstract
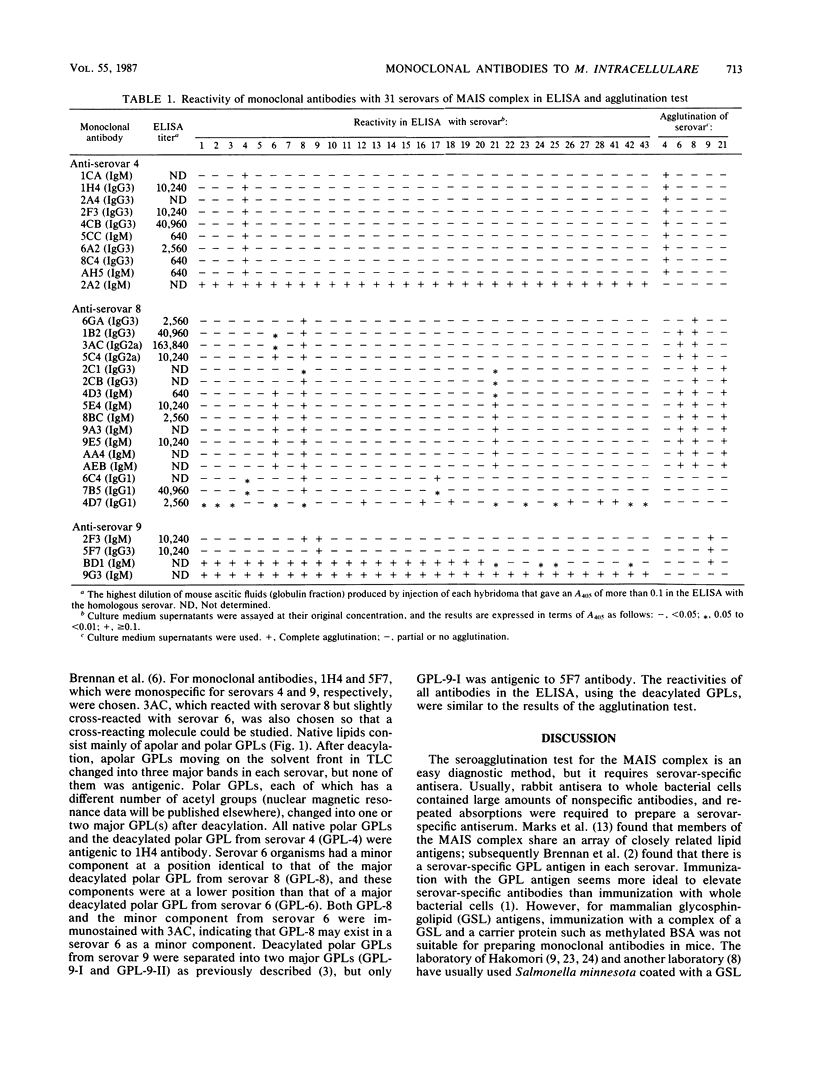
Serovar-specific monoclonal antibodies against Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex serovars 4, 8, and 9 were prepared. Nine, four, and one monoclonal antibodies, respectively, to the serovars were prepared by the usual cell fusion technique. All nine monoclonal antibodies to serovar 4 were monospecific for their homologous serovar and reacted with several native glycopeptidolipids (GPLs) and one major deacylated GPL from the homologous serovar. One of the four monoclonal antibodies to serovar 8 seemed to be monospecific for its homologous serovar, but the other cross-reacted with serovar 6 because serovar 6 organisms contain the same components as does the major deacylated GPL from serovar 8. One monoclonal antibody to serovar 9 was monospecific for its homologous serovar and reacted with one of the two major deacylated GPLs from this serovar. These antibody preparations proved useful for serovar identification.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow W. W., Brennan P. J. Immunogenicity of type-specific C-mycoside glycopeptidolipids of mycobacteria. Infect Immun. 1982 May;36(2):678–684. doi: 10.1128/iai.36.2.678-684.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Brennan P. J., Heifets M., Ullom B. P. Thin-layer chromatography of lipid antigens as a means of identifying nontuberculous mycobacteria. J Clin Microbiol. 1982 Mar;15(3):447–455. doi: 10.1128/jcm.15.3.447-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Mayer H., Aspinall G. O., Nam Shin J. E. Structures of the glycopeptidolipid antigens from serovars in the Mycobacterium avium/Mycobacterium intracellulare/Mycobacterium scrofulaceum serocomplex. Eur J Biochem. 1981 Mar 16;115(1):7–15. doi: 10.1111/j.1432-1033.1981.tb06190.x. [DOI] [PubMed] [Google Scholar]
- Brennan P. J., Souhrada M., Ullom B., McClatchy J. K., Goren M. B. Identification of atypical mycobacteria by thin-layer chromatography of their surface antigens. J Clin Microbiol. 1978 Oct;8(4):374–379. doi: 10.1128/jcm.8.4.374-379.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Varki A. P., Varki N. M., Stallcup W. B., Levine J., Reisfeld R. A. A monoclonal antibody recognizes an O-acylated sialic acid in a human melanoma-associated ganglioside. J Biol Chem. 1984 Jun 25;259(12):7453–7459. [PubMed] [Google Scholar]
- Fredman P., Jeansson S., Lycke E., Svennerholm L. A monoclonal antibody reacting specifically with ganglioside GD1b in human brain. FEBS Lett. 1985 Sep 9;189(1):23–26. doi: 10.1016/0014-5793(85)80834-6. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Nudelman E., Levery S. B., Patterson C. M. Human cancer-associated gangliosides defined by a monoclonal antibody (IB9) directed to sialosyl alpha 2 leads to 6 galactosyl residue: a preliminary note. Biochem Biophys Res Commun. 1983 Jun 29;113(3):791–798. doi: 10.1016/0006-291x(83)91069-0. [DOI] [PubMed] [Google Scholar]
- Higashi H., Fukui Y., Ueda S., Kato S., Hirabayashi Y., Matsumoto M., Naiki M. Sensitive enzyme-immunostaining and densitometric determination on thin-layer chromatography of N-glycolylneuraminic acid-containing glycosphingolipids, Hanganutziu-Deicher antigens. J Biochem. 1984 May;95(5):1517–1520. doi: 10.1093/oxfordjournals.jbchem.a134760. [DOI] [PubMed] [Google Scholar]
- Kannagi R., Cochran N. A., Ishigami F., Hakomori S., Andrews P. W., Knowles B. B., Solter D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2(12):2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J., Jenkins P. A., Schaefer W. B. Thin-layer chromatography of mycobacterial lipids as an aid to classification: technical improvements: mycobacterium avium, M. intracellulare (Battey bacilli). Tubercle. 1971 Sep;52(3):219–225. doi: 10.1016/0041-3879(71)90044-4. [DOI] [PubMed] [Google Scholar]
- Masaki S., Shimizu K., Cho N., Hirose T. Isolation of mycobacteria from lymph nodes of pigs and their environment. Nihon Juigaku Zasshi. 1982 Apr;44(2):213–221. doi: 10.1292/jvms1939.44.213. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Higashi H., Hirabayashi Y., Kato S., Naiki M. Detection of 4-O-acetyl-N-glycolylneuraminyl lactosylceramide as one of tumor-associated antigens in human colon cancer tissues by specific antibody. Mol Immunol. 1986 Jun;23(6):631–638. doi: 10.1016/0161-5890(86)90100-8. [DOI] [PubMed] [Google Scholar]
- Naiki M., Kato M. Immunological identification of blood group Pk antigen on normal human erythrocytes and isolation of anti-Pk with different affinity. Vox Sang. 1979;37(1):30–38. doi: 10.1111/j.1423-0410.1979.tb02265.x. [DOI] [PubMed] [Google Scholar]
- Orskov F., Orskov I., Sutton A., Schneerson R., Lin W., Egan W., Hoff G. E., Robbins J. B. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979 Mar 1;149(3):669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. B. Serologic identification and classification of the atypical mycobacteria by their agglutination. Am Rev Respir Dis. 1965 Dec;92(6):85–93. doi: 10.1164/arrd.1965.92.6P2.85. [DOI] [PubMed] [Google Scholar]
- TSUKAMURA M. Differentiationof mycobacterium tuberculosis from other mycobacteria by sodium salicylate susceptiblity. Am Rev Respir Dis. 1962 Jul;86:81–83. doi: 10.1164/arrd.1962.86.1.81. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- Yanagihara D. L., Barr V. L., Knisley C. V., Tsang A. Y., McClatchy J. K., Brennan P. J. Enzyme-linked immunosorbent assay of glycolipid antigens for identification of mycobacteria. J Clin Microbiol. 1985 Apr;21(4):569–574. doi: 10.1128/jcm.21.4.569-574.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. W., Jr, MacDonald E. M., Nowinski R. C., Hakomori S. I. Production of monoclonal antibodies specific for two distinct steric portions of the glycolipid ganglio-N-triosylceramide (asialo GM2). J Exp Med. 1979 Oct 1;150(4):1008–1019. doi: 10.1084/jem.150.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. W., Jr, Portoukalian J., Hakomori S. Two monoclonal anticarbohydrate antibodies directed to glycosphingolipids with a lacto-N-glycosyl type II chain. J Biol Chem. 1981 Nov 10;256(21):10967–10972. [PubMed] [Google Scholar]
- Yugi H., Nemoto H., Watanabe K. Senotypes of Mycobacterium intracellulare of porcine origin. Natl Inst Anim Health Q (Tokyo) 1972 Fall;12(3):168–169. [PubMed] [Google Scholar]



