Abstract
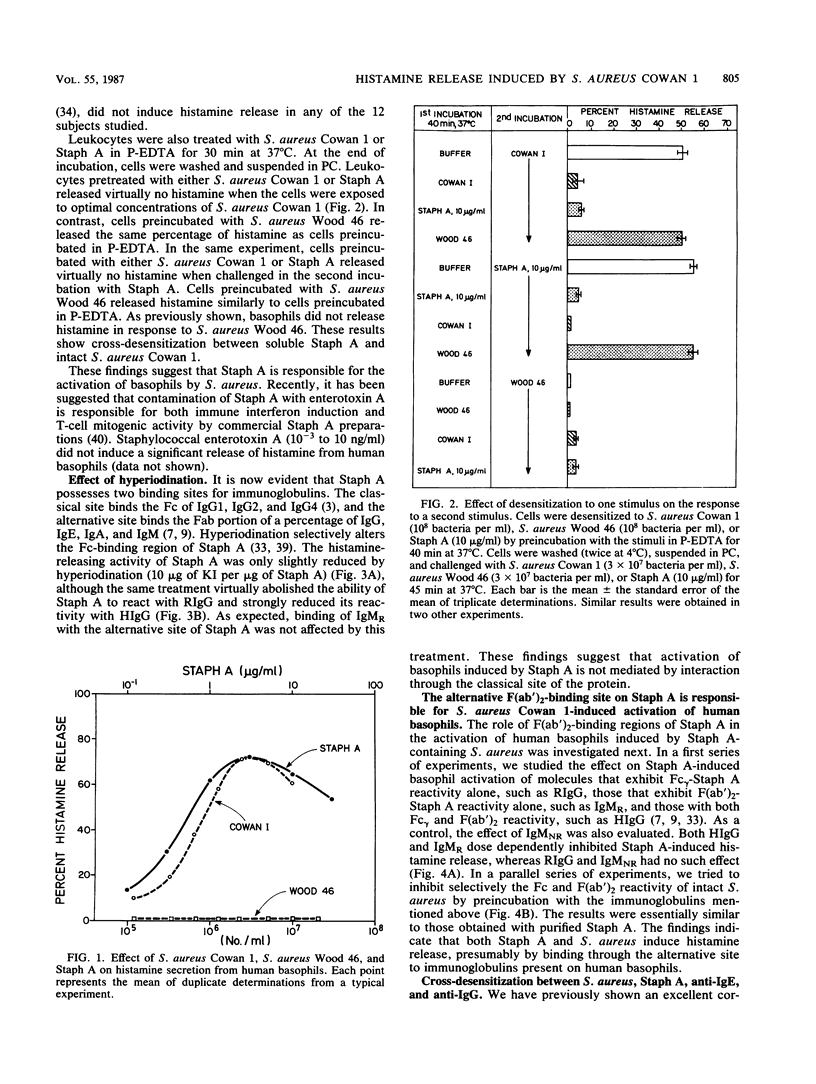
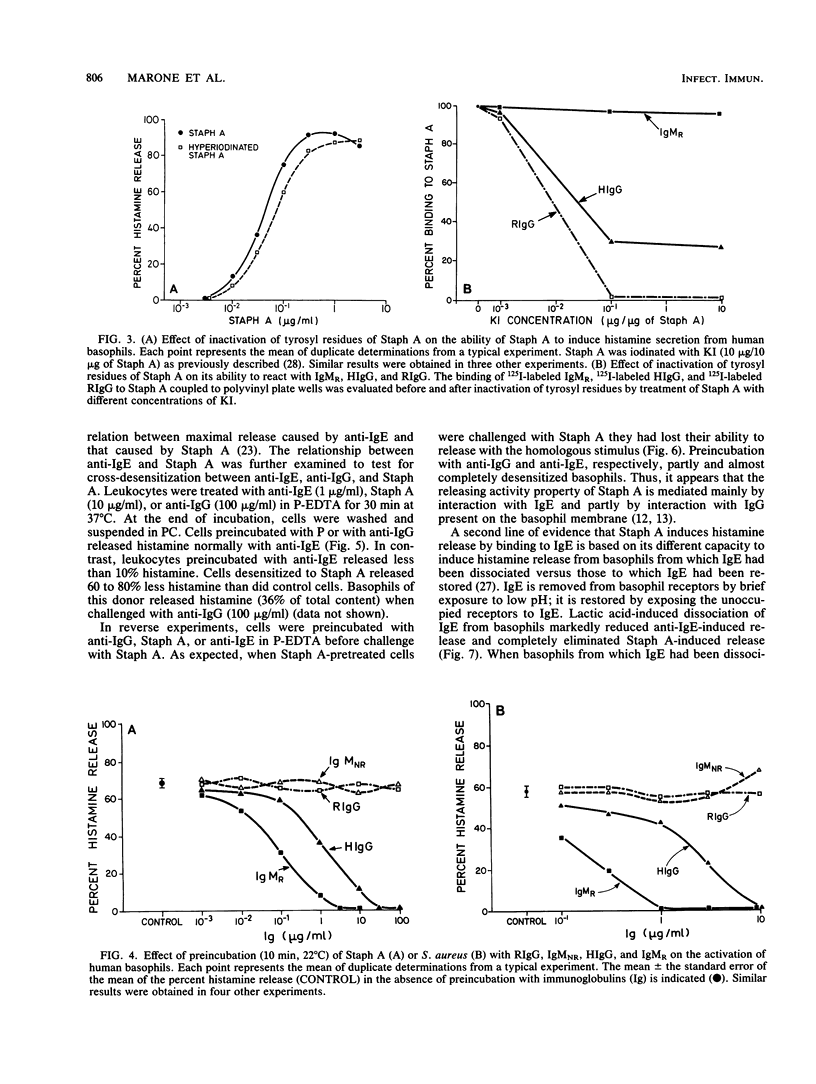
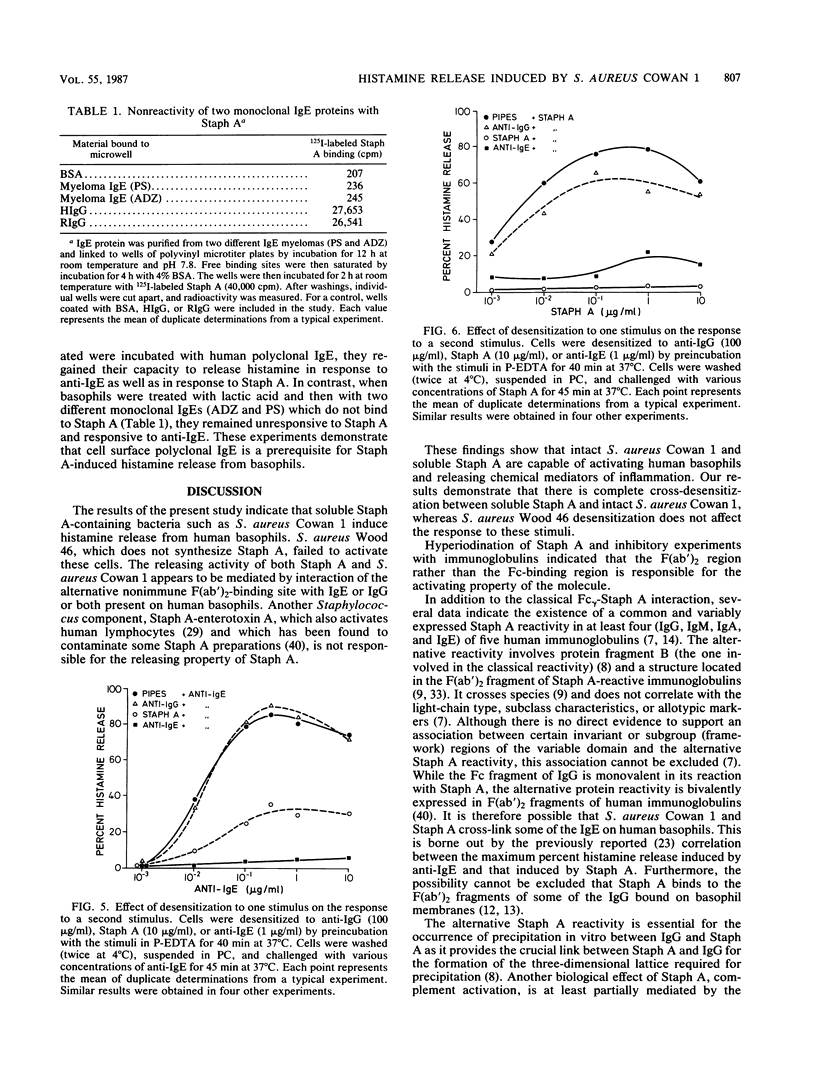
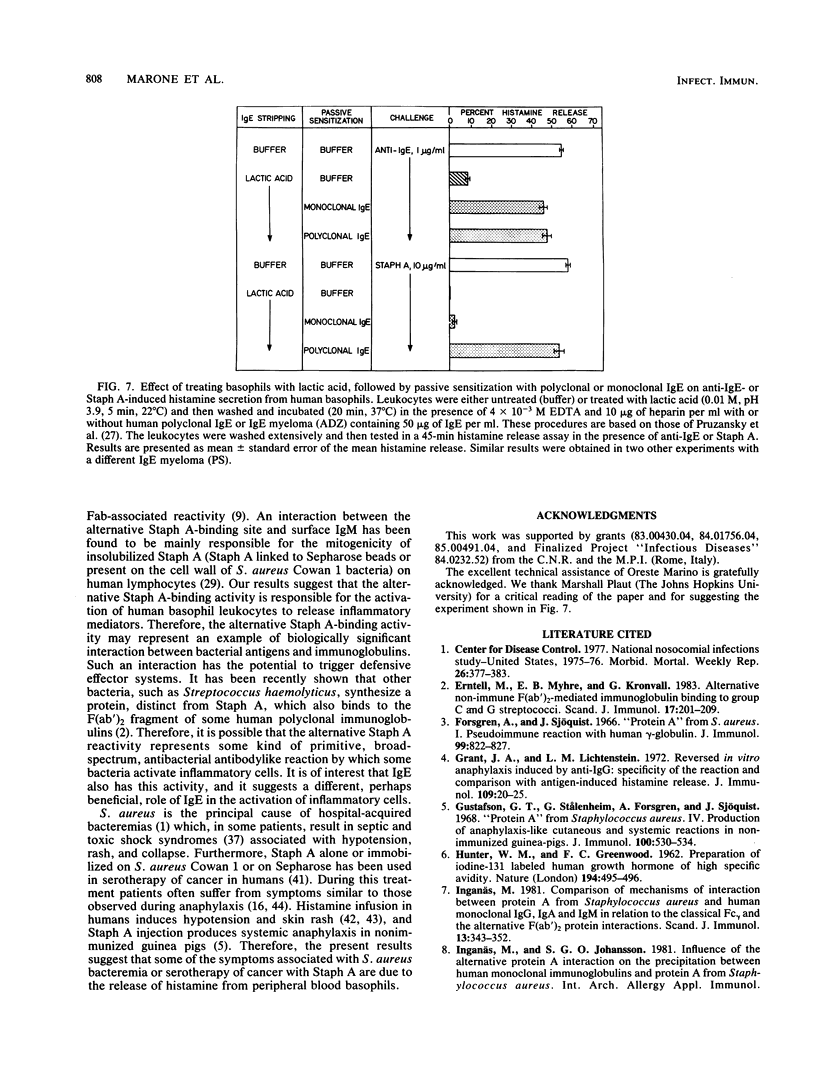
We investigated the capacity of Staphylococcus aureus Cowan 1 and S. aureus Wood 46 to induce histamine release from human basophils in vitro. S. aureus Cowan 1 (10(5) to 10(7)/ml), which synthesizes protein A (Staph A), stimulated the release of histamine from basophils, whereas S. aureus Wood 46 (10(5) to 2 X 10(7)/ml), which does not synthesize Staph A, did not induce histamine secretion. Soluble Staph A (10(-3) to 10 micrograms/ml), but not staphylococcal enterotoxin A, induced histamine secretion from human basophils. Staph A binds through its classical site to the Fc region of human immunoglobulin G (IgG) and through its alternative site to the Fab portion of the different human immunoglobulins. Hyperiodination of Staph A, which destroys over 90% of the original Fc reactivity without altering the Fab-binding site, did not alter the ability of the protein to induce histamine release. The stimulating effect of Staph A was dose dependently inhibited by preincubation with human polyclonal IgG (0.3 to 100 micrograms/ml) and a human monoclonal IgM (0.3 to 100 micrograms/ml) which have F(ab')-Staph A reactivity. In contrast, rabbit IgG, which possesses only Fc-Staph A reactivity, and a Staph A-unreactive human monoclonal IgM did not inhibit Staph A activity. Similar results were obtained with intact S. aureus Cowan 1. Preincubation with either Staph A or anti-IgE (rabbit anti-Fc epsilon) resulted in complete desensitization to a subsequent challenge with the homologous stimulus. Staph A and anti-IgE induced partial cross-densensitization to the heterologous stimulus. Cells preincubated with anti-IgG (rabbit anti-Fc gamma) lost a small but significant part of their ability to release with Staph A but did not lose their response to anti-IgE. Basophils from which IgE had been dissociated by brief exposure to lactic acid no longer released histamine in response to anti-IgE and Staph A. When basophils from which IgE had been dissociated were incubated with human polyclonal IgE, they regained their ability to induce histamine in response to Staph A and anti-IgE. In contrast, two monoclonal IgEs which do not bind to Staph A did not restore the basophil responsiveness to Staph A. Furthermore, there was complete cross-desensitization between soluble Staph A and S. aureus Cowan 1, while cells desensitized to S. aureus Wood 46 released normally with Staph A and S. aureus Cowan 1.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erntell M., Myhre E. B., Kronvall G. Alternative non-immune F(ab')2-mediated immunoglobulin binding to group C and G streptococci. Scand J Immunol. 1983 Mar;17(3):201–209. doi: 10.1111/j.1365-3083.1983.tb00783.x. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Grant J. A., Lichtenstein L. M. Reversed in vitro anaphylaxis induced by anti-IgG: specificity of the reaction and comparison with antigen-induced histamine release. J Immunol. 1972 Jul;109(1):20–25. [PubMed] [Google Scholar]
- Gustafson G. T., Stålenheim G., Forsgren A., Sjöquist J. "Protein A" from Staphylococcus aureus. IV. Production of anaphylaxis-like cutaneous and systemic reactions in non-immunized guinea-pigs. J Immunol. 1968 Mar;100(3):530–534. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Inganäs M. Comparison of mechanisms of interaction between protein A from Staphylococcus aureus and human monoclonal IgG, IgA and IgM in relation to the classical FC gamma and the alternative F(ab')2 epsilon protein A interactions. Scand J Immunol. 1981;13(4):343–352. doi: 10.1111/j.1365-3083.1981.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Inganäs M., Johansson S. G., Bennich H. H. Interaction of human polyclonal IgE and IgG from different species with protein A from Staphylococcus aureus: demonstration of protein-A-reactive sites located in the Fab'2 fragment of human IgG. Scand J Immunol. 1980;12(1):23–31. doi: 10.1111/j.1365-3083.1980.tb00037.x. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T., Lee E. H. Biologic function of the Fc fragments of E myeloma protein. Immunochemistry. 1970 Aug;7(8):687–702. doi: 10.1016/0019-2791(70)90175-8. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Tomioka H., Ishizaka T. Mechanisms of passive sensitization. I. Presence of IgE and IgG molecules on human leukocytes. J Immunol. 1970 Dec;105(6):1459–1467. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Johansson S. G., Bennich H. Histamine release from human leukocytes by anti-gamma E antibodies. J Immunol. 1969 Apr;102(4):884–892. [PubMed] [Google Scholar]
- Ishizaka T., Sterk A. R., Ishizaka K. Demonstration of Fcgamma receptors on human basophil granulocytes. J Immunol. 1979 Aug;123(2):578–583. [PubMed] [Google Scholar]
- Johansson S. G., Inganäs M. Interaction of polyclonal human IgE with protein-A from Staphylococcus aureus. Immunol Rev. 1978;41:248–260. doi: 10.1111/j.1600-065x.1978.tb01467.x. [DOI] [PubMed] [Google Scholar]
- Lind I., Harboe M., Folling I. Protein A reactivity of two distinct groups of human monoclonal IgM. Scand J Immunol. 1975;4(8):843–848. doi: 10.1111/j.1365-3083.1975.tb03726.x. [DOI] [PubMed] [Google Scholar]
- MacKintosh F. R., Bennett K., Schiff S., Shields J., Hall S. W. Treatment of advanced malignancy with plasma perfused over staphylococcal protein A. West J Med. 1983 Jul;139(1):36–40. [PMC free article] [PubMed] [Google Scholar]
- Marone G., Columbo M., Poto S., Condorelli M. Inhibition of histamine release from human basophils in vitro by calmodulin antagonists. Clin Immunol Immunopathol. 1983 Sep;28(3):334–340. doi: 10.1016/0090-1229(83)90100-9. [DOI] [PubMed] [Google Scholar]
- Marone G., Columbo M., Soppelsa L., Condorelli M. The mechanism of basophil histamine release induced by pepstatin A. J Immunol. 1984 Sep;133(3):1542–1546. [PubMed] [Google Scholar]
- Marone G., Findlay S. R., Lichtenstein L. M. Modulation of histamine release from human basophils in vitro by physiological concentrations of zinc. J Pharmacol Exp Ther. 1981 May;217(2):292–298. [PubMed] [Google Scholar]
- Marone G., Giugliano R., Lembo G., Ayala F. Human basophil releasability. II. Changes in basophil releasability in patients with atopic dermatitis. J Invest Dermatol. 1986 Jul;87(1):19–23. doi: 10.1111/1523-1747.ep12523520. [DOI] [PubMed] [Google Scholar]
- Marone G., Kagey-Sobotka A., Lichtenstein L. M. Effects of arachidonic acid and its metabolites on antigen-induced histamine release from human basophils in vitro. J Immunol. 1979 Oct;123(4):1669–1677. [PubMed] [Google Scholar]
- Marone G., Poto S., Petracca R., Triggiani M., de Lutio di Castelguidone E., Condorelli M. Activation of human basophils by staphylococcal protein A. I. The role of cyclic AMP, arachidonic acid metabolites, microtubules and microfilaments. Clin Exp Immunol. 1982 Dec;50(3):661–668. [PMC free article] [PubMed] [Google Scholar]
- Marone G., Poto S., di Martino L., Condorelli M. Human basophil releasability. I. Age-related changes in basophil releasability. J Allergy Clin Immunol. 1986 Feb;77(2):377–383. doi: 10.1016/s0091-6749(86)80121-x. [DOI] [PubMed] [Google Scholar]
- Marone G., Vigorita S., Antonelli C., Torella G., Genovese A., Condorelli M. Evidence for an adenosine A2/Ra receptor on human basophils. Life Sci. 1985 Jan 28;36(4):339–345. doi: 10.1016/0024-3205(85)90119-5. [DOI] [PubMed] [Google Scholar]
- Martin R. R., White A. The in vitro release of leukocyte histamine by staphylococcal antigens. J Immunol. 1969 Feb;102(2):437–441. [PubMed] [Google Scholar]
- Petersson B. A. Induction of histamine release and desensitization in human leukocytes. Scand J Immunol. 1975;4(8):777–784. doi: 10.1111/j.1365-3083.1975.tb03717.x. [DOI] [PubMed] [Google Scholar]
- Pruzansky J. J., Grammer L. C., Patterson R., Roberts M. Dissociation of IgE from receptors on human basophils. I. Enhanced passive sensitization for histamine release. J Immunol. 1983 Oct;131(4):1949–1953. [PubMed] [Google Scholar]
- Romagnani S., Almerigogna F., Giudizi G. M., Ricci M. Rosette formation with protein A-coated erythrocytes: a method for detecting both IgG-bearing cells and another subset of human peripheral blood B lymphocytes. J Immunol Methods. 1980;33(1):11–21. doi: 10.1016/0022-1759(80)90078-2. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Biagiotti R., Giudizi M. G., Almerigogna F., Alessi A., Ricci M. Protein A and enterotoxin A: two distinct Staphylococcus mitogens for human T lymphocytes. J Immunol. 1984 Feb;132(2):566–568. [PubMed] [Google Scholar]
- Romagnani S., Damiani G., Giudizi M. G., Biagiotti R., Almerigogna F., Delprete G. F., Maggi E., Bargellesi A., Ricci M. In vitro production of IgE by human peripheral blood mononuclear cells. III. Demonstration of a circulating IgE-bearing cell involved in the spontaneous IgE biosynthesis. Clin Exp Immunol. 1982 Jul;49(1):176–184. [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Almerigogna F., Ricci M. Interaction of staphylococcal protein A with membrane components of IgM- and/or IgD-bearing lymphocytes from human tonsil. J Immunol. 1980 Apr;124(4):1620–1626. [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., Biagiotti R., Almerigogna F., Maggi E., Del Prete G., Ricci M. Surface immunoglobulins are involved in the interaction of protein A with human B cells and in the triggering of B cell proliferation induced by protein A-containing Staphylococcus aureus. J Immunol. 1981 Oct;127(4):1307–1313. [PubMed] [Google Scholar]
- Romagnani S., Giudizi M. G., del Prete G., Maggi E., Biagiotti R., Almerigogna F., Ricci M. Demonstration on protein A of two distinct immunoglobulin-binding sites and their role in the mitogenic activity of Staphylococcus aureus Cowan I on human B cells. J Immunol. 1982 Aug;129(2):596–602. [PubMed] [Google Scholar]
- Rynnel-Dagö B., Ringdén O., Alfredsson H., Möller E. The use of bacteria for the functional characterization of human lymphocyte subpopulations in various lymphoid organs. Scand J Immunol. 1978;8(5):369–375. doi: 10.1111/j.1365-3083.1978.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Sala P., Tonutti E., Ruscio M., Colle R., Antonutto G., Falconieri G. IgE myeloma. Report of a new case and review of the literature. Haematologica. 1981 Dec;66(6):787–795. [PubMed] [Google Scholar]
- Saltvedt E., Harboe M. Binding of IgA to protein-A-containing staphylococci: relationship to subclasses. Scand J Immunol. 1976;5(10):1103–1108. doi: 10.1111/j.1365-3083.1976.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Sheagren J. N. Staphylococcus aureus. The persistent pathogen (second of two parts). N Engl J Med. 1984 May 31;310(22):1437–1442. doi: 10.1056/NEJM198405313102206. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974 Feb;57(2):383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- Sjöholm I., Bjerkén A., Sjöquist J. Protein A from Staphylococcus aureus. XIV. The effect of nitration of protein A with tetranitromethane and subsequent reduction. J Immunol. 1973 Jun;110(6):1562–1569. [PubMed] [Google Scholar]
- Smith E. M., Johnson H. M., Blalock J. E. Staphylococcus aureus protein A induces the production of interferon-alpha in human lymphocytes and interferon-alpha/beta in mouse spleen cells. J Immunol. 1983 Feb;130(2):773–776. [PubMed] [Google Scholar]
- Terman D. S., Young J. B., Shearer W. T., Ayus C., Lehane D., Mattioli C., Espada R., Howell J. F., Yamamoto T., Zaleski H. I. Preliminary observations of the effects on breast adenocarcinoma of plasma perfused over immobilized protein A. N Engl J Med. 1981 Nov 12;305(20):1195–1200. doi: 10.1056/NEJM198111123052006. [DOI] [PubMed] [Google Scholar]
- Vigorito C., Poto S., Picotti G. B., Triggiani M., Marone G. Effect of activation of the H1 receptor on coronary hemodynamics in man. Circulation. 1986 Jun;73(6):1175–1182. doi: 10.1161/01.cir.73.6.1175. [DOI] [PubMed] [Google Scholar]
- Vigorito C., Russo P., Picotti G. B., Chiariello M., Poto S., Marone G. Cardiovascular effects of histamine infusion in man. J Cardiovasc Pharmacol. 1983 Jul-Aug;5(4):531–537. doi: 10.1097/00005344-198307000-00004. [DOI] [PubMed] [Google Scholar]
- Young J. B., Ayus J. C., Miller L. K., Divine G. W., Frommer J. P., Miller R. R., Terman D. S. Cardiopulmonary toxicity in patients with breast carcinoma during plasma perfusion over immobilized protein A. Pathophysiology of reaction and attenuating methods. Am J Med. 1983 Aug;75(2):278–288. doi: 10.1016/0002-9343(83)91206-8. [DOI] [PubMed] [Google Scholar]


