Abstract
Somatic cell hybrids secreting monoclonal antibodies against the core-glycolipid portion of enterobacterial endotoxin were derived from mice immunized with Escherichia coli J5 or Salmonella minnesota R595 heat-killed organisms or lipopolysaccharide (LPS). Eight antibodies were selected for their ability to cross-react with several members of a panel of gram-negative bacterial antigens in a radioimmunoassay. This panel represented five genera and two families of organisms: E. coli O111:B4, E. coli O55:B5, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella minnesota, and Serratia marcescens. The binding sites for six of the antibodies were unequivocally localized within the lipid A moiety of the endotoxin molecule by using the radioimmunoassay on LPS and free lipid A. The anti-lipid A antibodies were further characterized for their ability to interact with LPS variants by using a sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunostaining procedure. The monoclonal antibodies bound almost exclusively to the low-molecular-weight species of LPS on the polyacrylamide gel. These components corresponded to LPS isolated from rough strains of organisms (strains which lack O-specific carbohydrate). These results suggested that the cross-reactive component of antisera raised against rough mutants of gram-negative bacteria contain antibodies of lipid A specificity. Moreover, the determinant within the lipid A moiety of LPS may have been accessible to the monoclonal antibodies only in those endotoxin molecules on the outer membrane surface which lack the O-specific carbohydrate.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braude A. I., Douglas H. Passive immunization against the local Shwartzman reaction. J Immunol. 1972 Feb;108(2):505–512. [PubMed] [Google Scholar]
- Braude A. I., Ziegler E. J., Douglas H., McCutchan J. A. Antibody to cell wall glycolipid of Gram-negative bacteria: induction of immunity to bacteremia and endotoxemia. J Infect Dis. 1977 Aug;136 (Suppl):S167–S173. doi: 10.1093/infdis/136.supplement.s167. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Bogard W. C., Jr, Cerra F. B. Efficacy of type-specific and cross-reactive murine monoclonal antibodies directed against endotoxin during experimental sepsis. Surgery. 1985 Aug;98(2):283–290. [PubMed] [Google Scholar]
- Dunn D. L., Ferguson R. M. Immunotherapy of gram-negative bacterial sepsis: enhanced survival in a guinea pig model by use of rabbit antiserum to Escherichia coli J5. Surgery. 1982 Aug;92(2):212–219. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Jay F., Nerkar D., Veleva K., Brade H., Strittmatter W. Immunogenic properties of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):546–552. doi: 10.1093/clinids/6.4.546. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Shenep J. L. Failure of monoclonal antibodies to core glycolipid to bind intact smooth strains of Escherichia coli. J Infect Dis. 1985 Jun;151(6):1005–1011. doi: 10.1093/infdis/151.6.1005. [DOI] [PubMed] [Google Scholar]
- Greisman S. E., DuBuy J. B., Woodward C. L. Experimental gram-negative bacterial sepsis: reevaluation of the ability of rough mutant antisera to protect mice. Proc Soc Exp Biol Med. 1978 Jul;158(3):482–490. doi: 10.3181/00379727-158-40231. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Johns M. A., Bruins S. C., McCabe W. R. Immunization with R mutants of Salmonella minnesota. II. Serological response to lipid A and the lipopolysaccharide of Re mutants. Infect Immun. 1977 Jul;17(1):9–15. doi: 10.1128/iai.17.1.9-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns M., Skehill A., McCabe W. R. Immunization with rough mutants of Salmonella minnesota. IV. Protection by antisera to O and rough antigens against endotoxin. J Infect Dis. 1983 Jan;147(1):57–67. doi: 10.1093/infdis/147.1.57. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Khaw B. A., Mattis J. A., Melincoff G., Strauss H. W., Gold H. K., Haber E. Monoclonal antibody to cardiac myosin: imaging of experimental myocardial infarction. Hybridoma. 1984;3(1):11–23. doi: 10.1089/hyb.1984.3.11. [DOI] [PubMed] [Google Scholar]
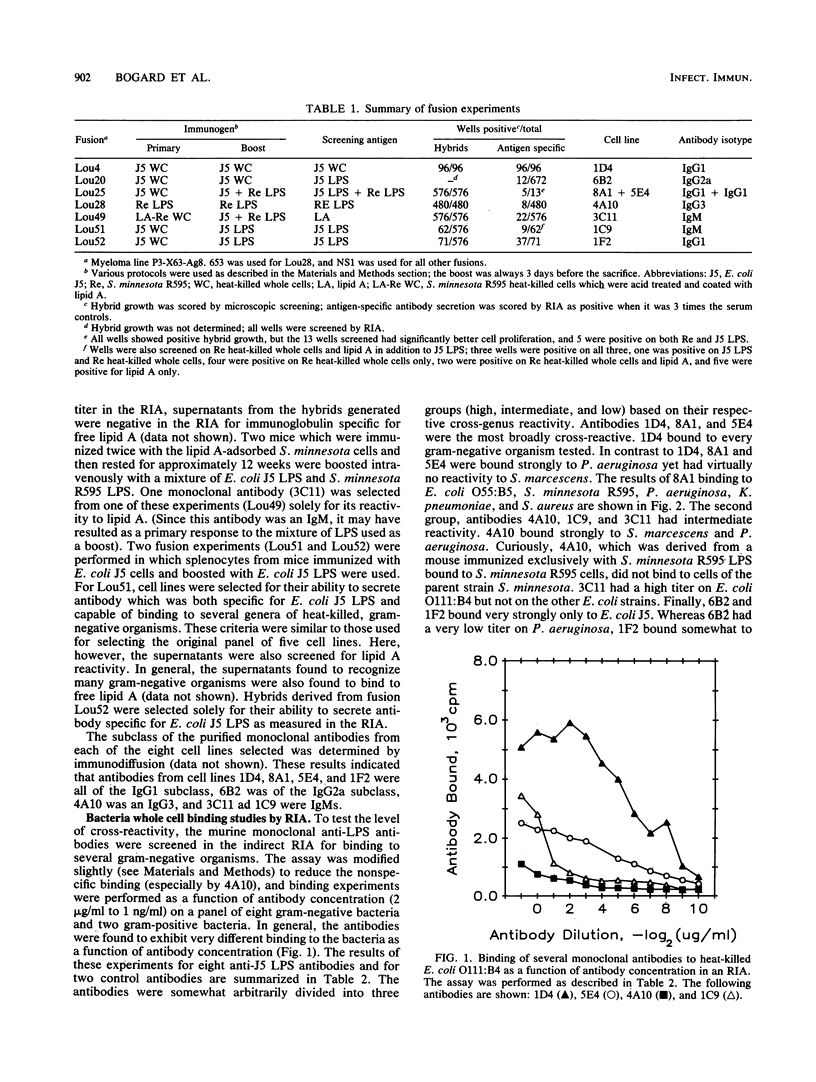
- Kirkland T. N., Colwell D. E., Michalek S. M., McGhee J. R., Ziegler E. J. Analysis of the fine specificity and cross-reactivity of monoclonal anti-lipid A antibodies. J Immunol. 1986 Dec 1;137(11):3614–3619. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Mattsby-Baltzer I., Claësson I., Hanson L. A., Jodal U., Kaijser B., Lindberg U., Peterson H. Antibodies to lipid A during urinary tract infection. J Infect Dis. 1981 Oct;144(4):319–328. doi: 10.1093/infdis/144.4.319. [DOI] [PubMed] [Google Scholar]
- McCabe W. R., Bruins S. C., Craven D. E., Johns M. Cross-reactive antigens: their potential for immunization-induced immunity to Gram-negative bacteria. J Infect Dis. 1977 Aug;136 (Suppl):S161–S166. doi: 10.1093/infdis/136.supplement.s161. [DOI] [PubMed] [Google Scholar]
- McCabe W. R. Immunization with R mutants of S. Minnesota. I. Protection against challenge with heterologous gram-negative bacilli. J Immunol. 1972 Mar;108(3):601–610. [PubMed] [Google Scholar]
- Miner K. M., Manyak C. L., Williams E., Jackson J., Jewell M., Gammon M. T., Ehrenfreund C., Hayes E., Callahan L. T., 3rd, Zweerink H. Characterization of murine monoclonal antibodies to Escherichia coli J5. Infect Immun. 1986 Apr;52(1):56–62. doi: 10.1128/iai.52.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Mullan N. A., Newsome P. M., Cunnington P. G., Palmer G. H., Wilson M. E. Protection against gram-negative infections with antiserum to lipid A from Salmonella minnesota R595. Infect Immun. 1974 Dec;10(6):1195–1201. doi: 10.1128/iai.10.6.1195-1201.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Crockford G., Bogard W. C., Jr, Hancock R. E. Monoclonal antibodies specific for Escherichia coli J5 lipopolysaccharide: cross-reaction with other gram-negative bacterial species. Infect Immun. 1984 Sep;45(3):631–636. doi: 10.1128/iai.45.3.631-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles M. J., Niswander C. A. Mouse monoclonal antibodies reactive with J5 lipopolysaccharide exhibit extensive serological cross-reactivity with a variety of gram-negative bacteria. Infect Immun. 1984 Dec;46(3):677–681. doi: 10.1128/iai.46.3.677-681.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng A. K., Chen C. L., Chang C. M., Nowotny A. Relationship of structure to function in bacterial endotoxins: serologically cross-reactive components and their effect on protection of mice against some gram-negative infections. J Gen Microbiol. 1976 May;94(1):107–116. doi: 10.1099/00221287-94-1-107. [DOI] [PubMed] [Google Scholar]
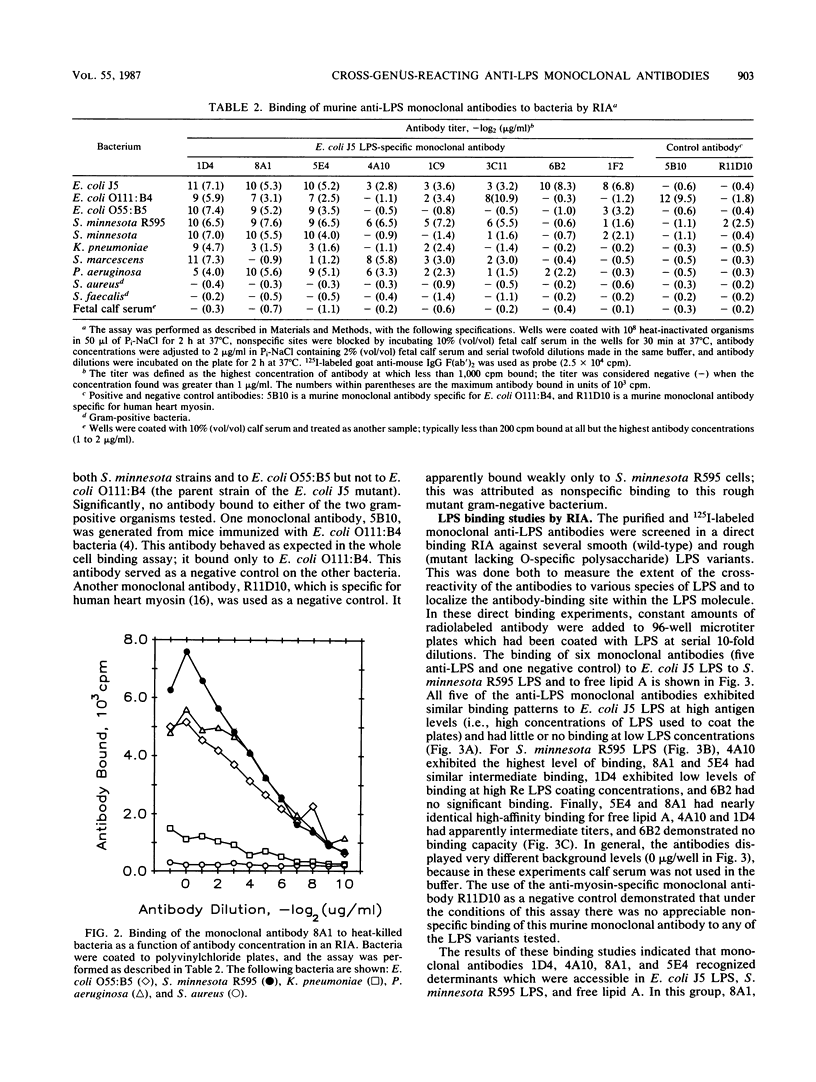
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Pennington J. E., Menkes E. Type-specific vs. cross-protective vaccination for gram-negative bacterial pneumonia. J Infect Dis. 1981 Dec;144(6):599–603. doi: 10.1093/infdis/144.6.599. [DOI] [PubMed] [Google Scholar]
- Peterson A. A., McGroarty E. J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J Bacteriol. 1985 May;162(2):738–745. doi: 10.1128/jb.162.2.738-745.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Huang A. I., Prescott R. K., Young L. S., Hunter K. W., Cruess D. F., Tsai C. M. Enhanced survival in Pseudomonas aeruginosa septicemia associated with high levels of circulating antibody to Escherichia coli endotoxin core. J Clin Invest. 1983 Dec;72(6):1874–1881. doi: 10.1172/JCI111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakulramrung R., Domingue G. J. Cross-reactive immunoprotective antibodies to Escherichia coli O111 rough mutant J5. J Infect Dis. 1985 Jun;151(6):995–1004. doi: 10.1093/infdis/151.6.995. [DOI] [PubMed] [Google Scholar]
- Tate W. J., 3rd, Douglas H., Braude A. I., Wells W. W. Protection against lethality of E. coli endotoxin with "O" antiserum. Ann N Y Acad Sci. 1966 Jun 30;133(2):746–762. doi: 10.1111/j.1749-6632.1966.tb52403.x. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- van Dijk W. C., Verbrugh H. A., van Erne-van der Tol M. E., Peters R., Verhoef J. Escherichia coli antibodies in opsonisation and protection against infection. J Med Microbiol. 1981 Nov;14(4):381–389. doi: 10.1099/00222615-14-4-381. [DOI] [PubMed] [Google Scholar]