Abstract
Twenty-three monoclonal antibodies (MAbs) prepared in seven different laboratories were studied, all of which recognized the 65-kilodalton (kDa) protein of Mycobacterium leprae as determined by Western blotting or gel radioimmunoassay or both. Fourteen of the MAbs recognized different epitopes, as evaluated by cross-competition studies using radiolabeled MAb and unlabeled inhibitors; the species specificity of these epitopes was defined by nitrocellulose dot blot immunoassays with bacterial sonic extract antigen preparations from 23 species of mycobacteria. Each of the 14 distinct MAbs recognized a 65-kDa protein produced by a lysogenized Escherichia coli Y1089 host containing cloned rDNA which included the gene for the M. leprae 65-kDa protein. Of the 14 distinct MAbs, 1 recognized an epitope found only on M. leprae, and the others recognized epitopes present on as few as 8 or as many as all 23 of the mycobacterial species studied. Identification of these distinct 65-kDa protein epitopes and use of the MAbs which recognize them should assist future structural studies of this protein and characterization of the T-cell reactive and serodiagnostically useful portions of the molecule.
Full text
PDF
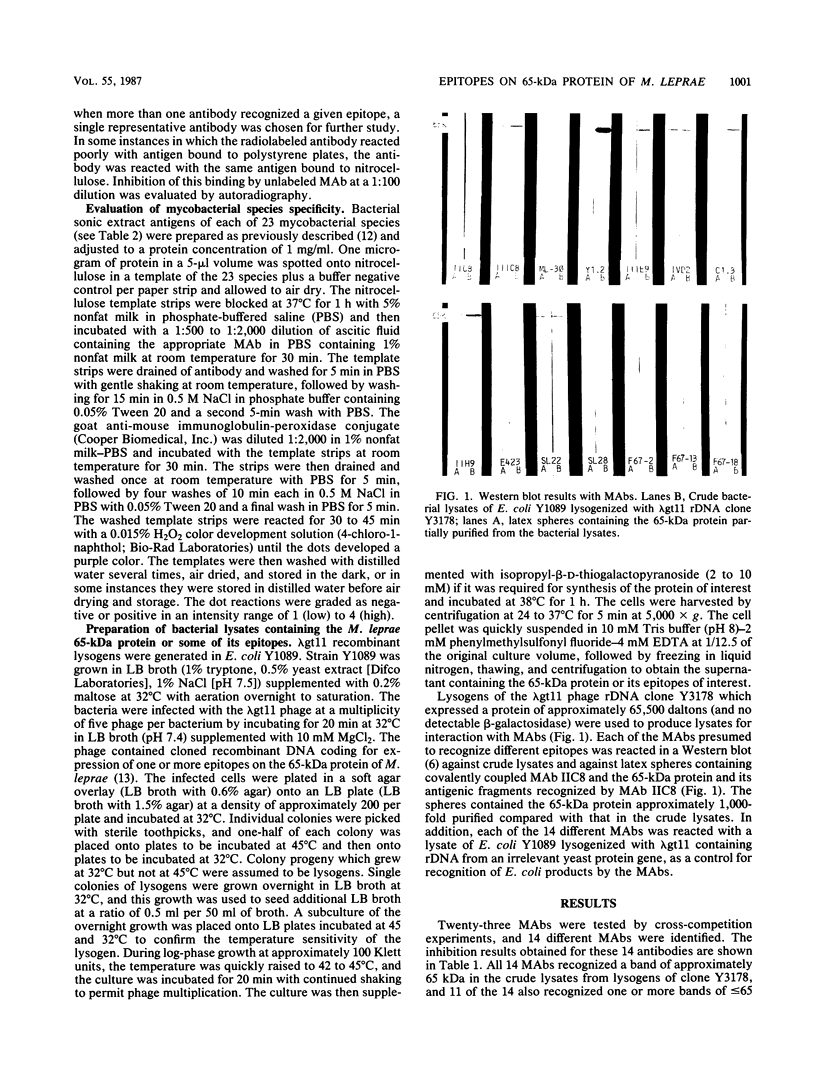
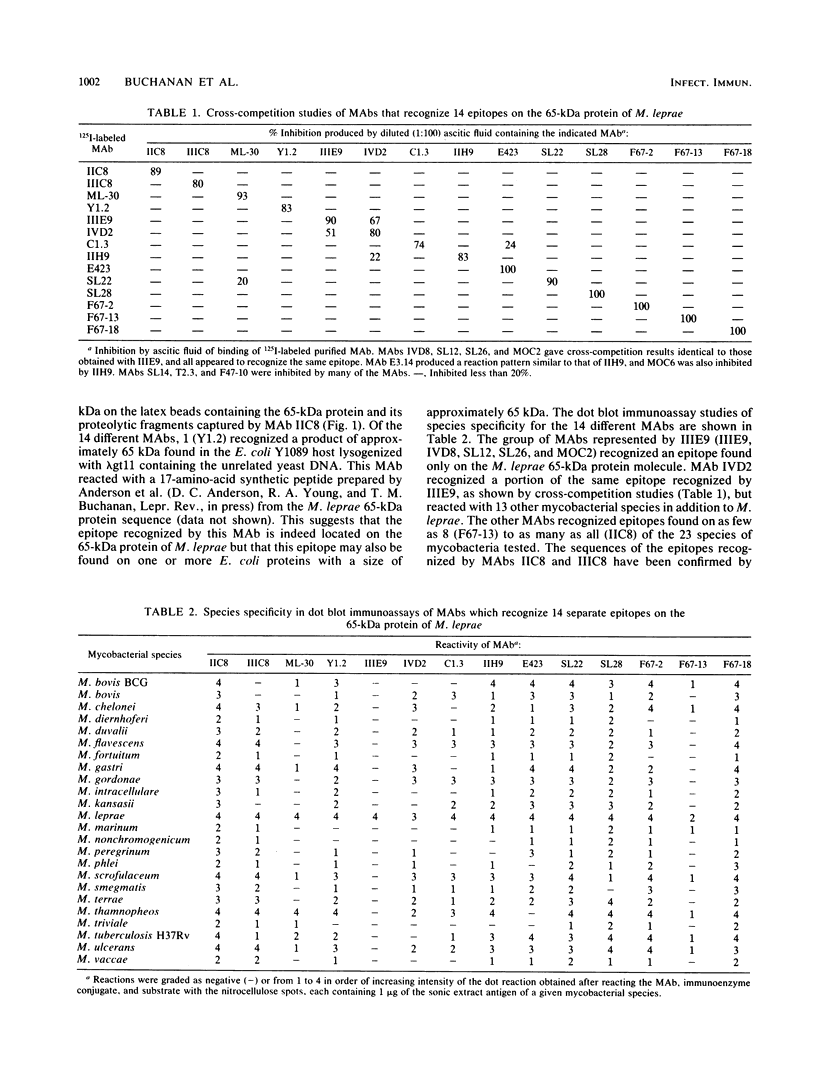

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Buchanan T. M. Production and partial characterization of monoclonal antibodies to Mycobacterium leprae. Infect Immun. 1982 Jul;37(1):172–178. doi: 10.1128/iai.37.1.172-178.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Miller R. A., Young D. B., Khanolkar S. R., Buchanan T. M. Immunochemical characterization of a protein associated with Mycobacterium leprae cell wall. Infect Immun. 1985 Aug;49(2):371–377. doi: 10.1128/iai.49.2.371-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Olsen D. A., Buchanan T. M. Analysis of the antigen specificity of the human serum immunoglobulin G immune response to complicated gonococcal infection. Infect Immun. 1984 Feb;43(2):706–709. doi: 10.1128/iai.43.2.706-709.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Sinha S., Aston R., Cussell D., Keen M., Sengupta U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983 Jun;52(3):528–536. [PMC free article] [PubMed] [Google Scholar]
- Levis W. R., Meeker H. C., Schuller-Levis G. B., Gillis T. P., Marino L. J., Jr, Zabriskie J. Serodiagnosis of leprosy: relationships between antibodies to Mycobacterium leprae phenolic glycolipid I and protein antigens. J Clin Microbiol. 1986 Dec;24(6):917–921. doi: 10.1128/jcm.24.6.917-921.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman J. T., Buchanan T. M. Monoclonal antibody activity against native and denatured forms of gonococcal outer membrane proteins as detected within ultrathin, longitudinal slices of polyacrylamide gels. J Immunol Methods. 1984 Dec 31;75(2):265–274. doi: 10.1016/0022-1759(84)90110-8. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Fohn M. J., Khanolkar S. R., Buchanan T. M. Monoclonal antibodies to a 28,000 mol. wt protein antigen of Mycobacterium leprae. Clin Exp Immunol. 1985 Jun;60(3):546–552. [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]



