Abstract
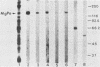
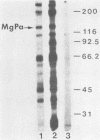
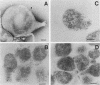
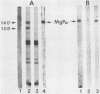
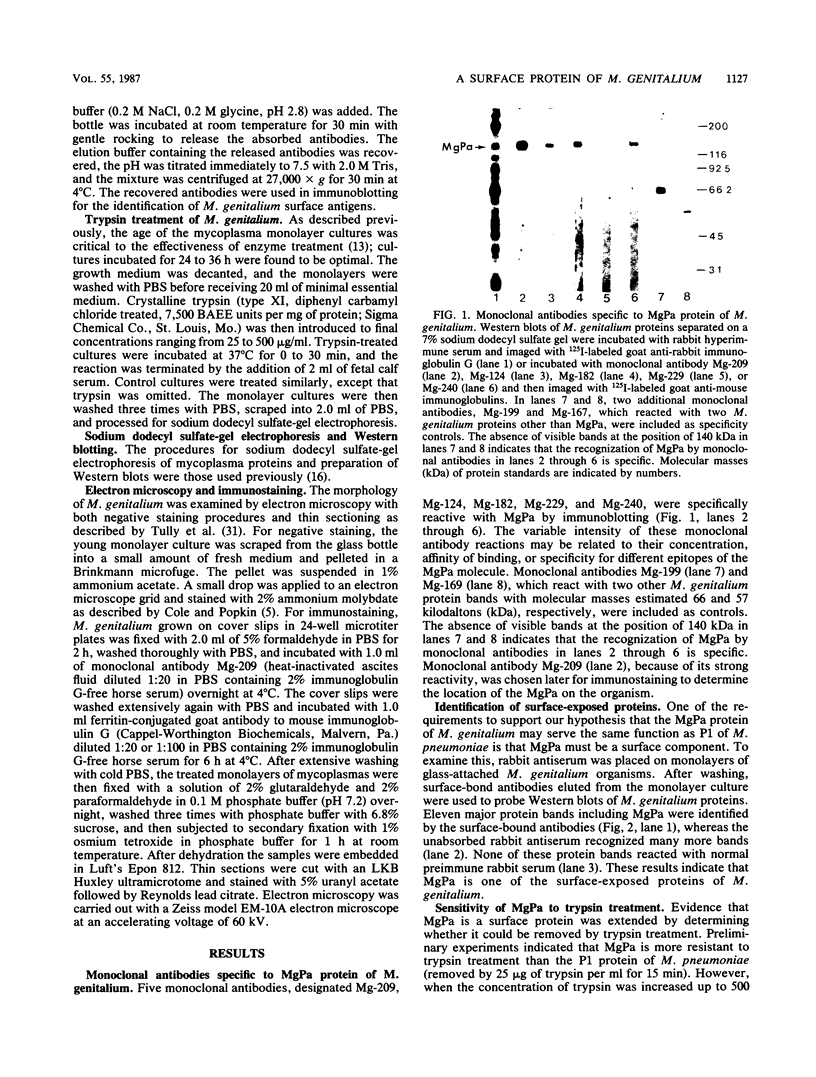
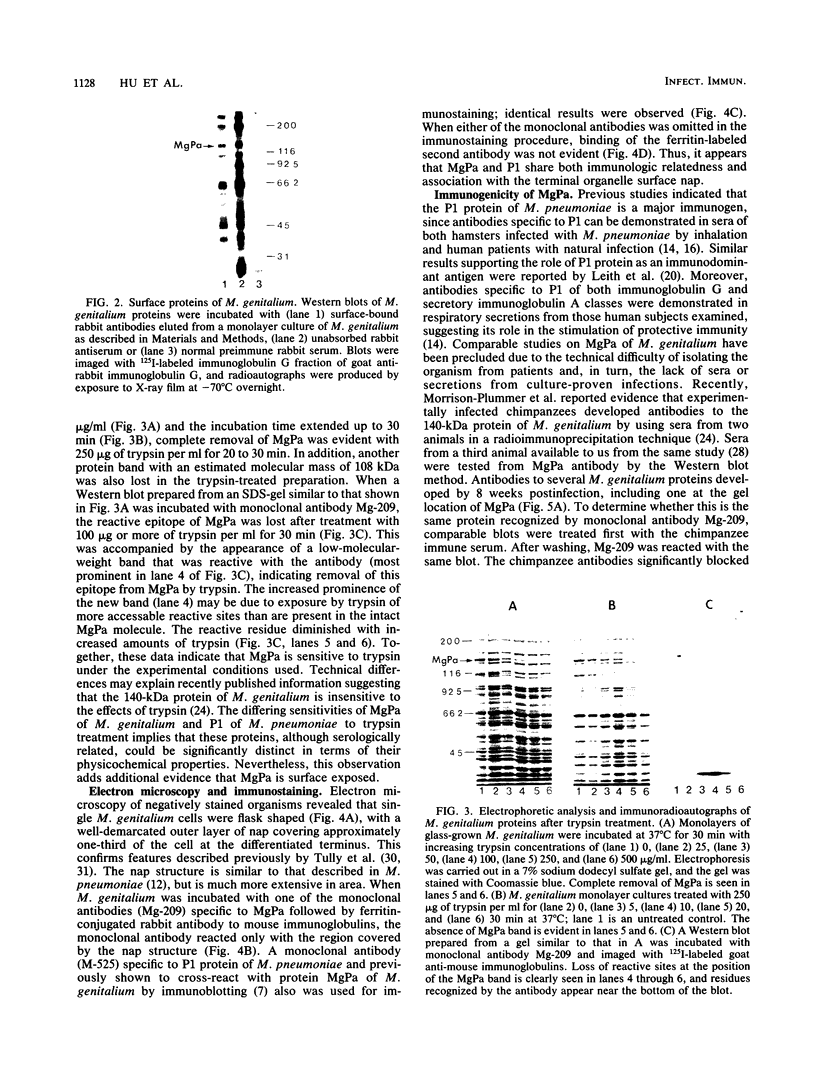
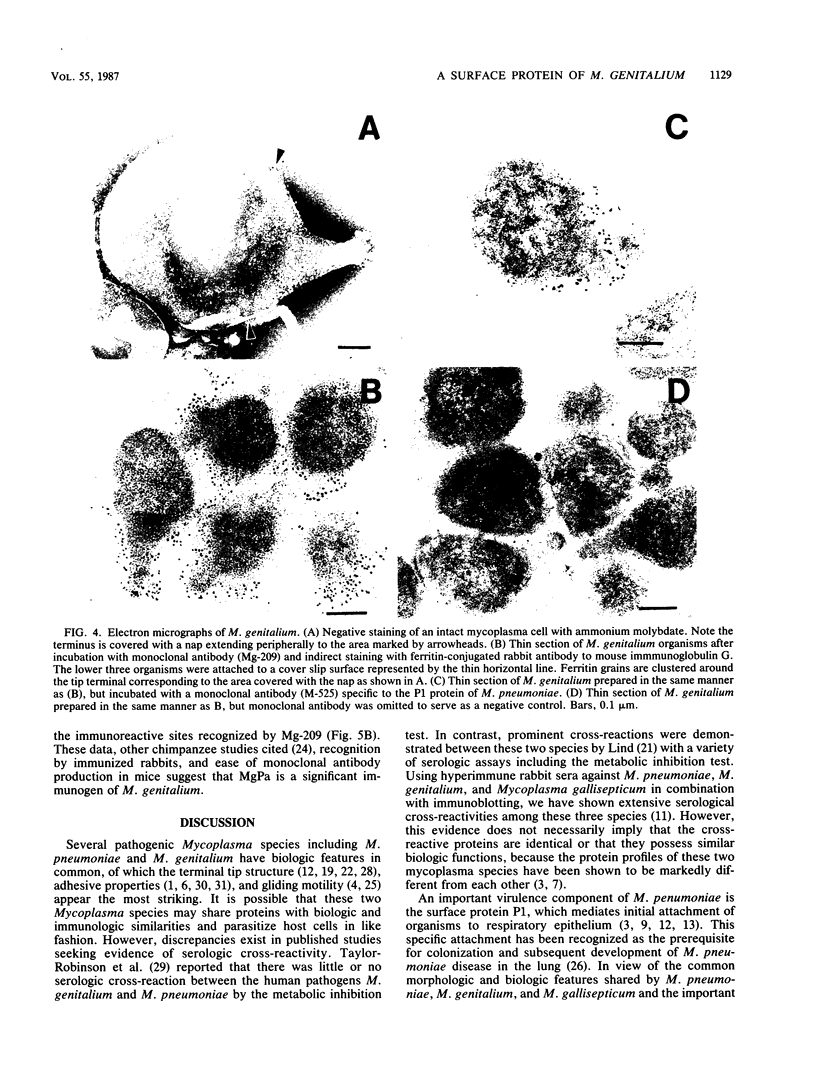
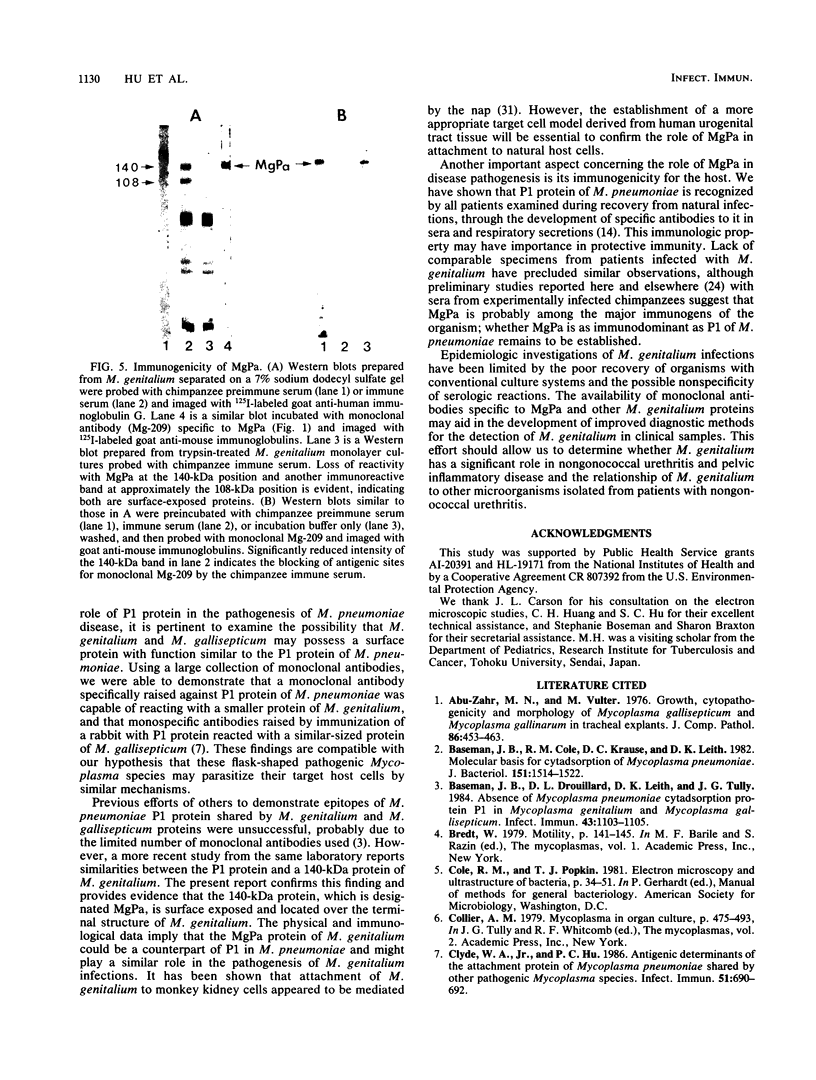
In previous studies with hyperimmune rabbit sera and monoclonal antibodies against the P1 protein of Mycoplasma pneumoniae, we obtained evidence of a shared antigenic determinant with a single protein of Mycoplasma genitalium. Because of biologic and morphologic similarities between these two human Mycoplasma species, attempts were made to characterize this cross-reacting protein of M. genitalium (designated MgPa). The protein was surface exposed and had an estimated molecular size of 140 kilodaltons. Electron microscopy with monoclonal antibodies produced against either MgPa or P1 demonstrated that MgPa is located over the surface of the terminal structure of M. genitalium which is covered by a nap layer. These immunologic and morphologic findings suggest that the MgPa protein of M. genitalium could be the counterpart of the P1 protein of M. pneumoniae.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Zahr M. N., Butler M. Growth, cytopathogenicity and morphology of Mycoplasma gallisepticum and M. gallinarum in tracheal explants. J Comp Pathol. 1976 Jul;86(3):455–463. doi: 10.1016/0021-9975(76)90014-1. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Drouillard D. L., Leith D. K., Tully J. G. Absence of Mycoplasma pneumoniae cytadsorption protein P1 in Mycoplasma genitalium and Mycoplasma gallisepticum. Infect Immun. 1984 Mar;43(3):1103–1105. doi: 10.1128/iai.43.3.1103-1105.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde W. A., Jr, Hu P. C. Antigenic determinants of the attachment protein of Mycoplasma pneumoniae shared by other pathogenic Mycoplasma species. Infect Immun. 1986 Feb;51(2):690–692. doi: 10.1128/iai.51.2.690-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleberg N. C., Pearlman E., Eisenstein B. I. Legionella pneumophila surface antigens cloned and expressed in Escherichia coli are translocated to the host cell surface and interact with specific anti-Legionella antibodies. J Bacteriol. 1984 Oct;160(1):199–203. doi: 10.1128/jb.160.1.199-203.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Herbrink P., Van Bussel F. J., Warnaar S. O. The antigen spot test (AST): a highly sensitive assay for the detection of antibodies. J Immunol Methods. 1982;48(3):293–298. doi: 10.1016/0022-1759(82)90330-1. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Clyde W. A., Jr, Collier A. M. Conservation of pathogenic mycoplasma antigens. Isr J Med Sci. 1984 Oct;20(10):916–919. [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Huang C. H., Collier A. M., Clyde W. A., Jr Demonstration of antibodies to Mycoplasma pneumoniae attachment protein in human sera and respiratory secretions. Infect Immun. 1983 Jul;41(1):437–439. doi: 10.1128/iai.41.1.437-439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Huang C. H., Huang Y. S., Collier A. M., Clyde W. A., Jr Demonstration of multiple antigenic determinants on Mycoplasma pneumoniae attachment protein by monoclonal antibodies. Infect Immun. 1985 Oct;50(1):292–296. doi: 10.1128/iai.50.1.292-296.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Huang Y. S., Graham J. A., Gardner D. E. Identification of immunogens of Mycoplasma pneumoniae by protein blotting. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1363–1370. doi: 10.1016/0006-291x(81)90273-4. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Powell D. A., Albright F., Gardner D. E., Collier A. M., Clyde W. A., Jr A solid-phase radioimmunoassay for detection of antibodies against Mycoplasma pneumoniae. J Clin Lab Immunol. 1983 Aug;11(4):209–213. [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff H., Rosengarten R., Lotz W., Fischer M., Lopatta D. Flask-shaped mycoplasmas: properties and pathogenicity for man and animals. Isr J Med Sci. 1984 Sep;20(9):848–853. [PubMed] [Google Scholar]
- Leith D. K., Trevino L. B., Tully J. G., Senterfit L. B., Baseman J. B. Host discrimination of Mycoplasma pneumoniae proteinaceous immunogens. J Exp Med. 1983 Feb 1;157(2):502–514. doi: 10.1084/jem.157.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind K. Serological cross-reactions between "Mycoplasma genitalium" and M. pneumoniae. Lancet. 1982 Nov 20;2(8308):1158–1159. doi: 10.1016/s0140-6736(82)92809-4. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J., Barrnett R. J. Ultrastructure and Ribosomes of Mycoplasma gallisepticum. J Bacteriol. 1965 Jul;90(1):193–204. doi: 10.1128/jb.90.1.193-204.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison-Plummer J., Lazzell A., Baseman J. B. Shared epitopes between Mycoplasma pneumoniae major adhesin protein P1 and a 140-kilodalton protein of Mycoplasma genitalium. Infect Immun. 1987 Jan;55(1):49–56. doi: 10.1128/iai.55.1.49-56.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller B. R., Taylor-Robinson D., Furr P. M. Serological evidence implicating Mycoplasma genitalium in pelvic inflammatory disease. Lancet. 1984 May 19;1(8386):1102–1103. doi: 10.1016/s0140-6736(84)92511-x. [DOI] [PubMed] [Google Scholar]
- Radestock U., Bredt W. Motility of Mycoplasma pneumoniae. J Bacteriol. 1977 Mar;129(3):1495–1501. doi: 10.1128/jb.129.3.1495-1501.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Tully J. G., Barile M. F. Urethral infection in male chimpanzees produced experimentally by Mycoplasma genitalium. Br J Exp Pathol. 1985 Feb;66(1):95–101. [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Tully J. G., Furr P. M., Cole R. M., Rose D. L., Hanna N. F. Urogenital mycoplasma infections of man: a review with observations on a recently discovered mycoplasma. Isr J Med Sci. 1981 Jul;17(7):524–530. [PubMed] [Google Scholar]
- Tully J. G., Taylor-Robinson D., Cole R. M., Rose D. L. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981 Jun 13;1(8233):1288–1291. doi: 10.1016/s0140-6736(81)92461-2. [DOI] [PubMed] [Google Scholar]