Abstract
To gain insights into the functions of a viral RNA replicase, we have assembled in vitro and entirely from nonplant sources, a fully functional replicase complex of Tomato bushy stunt virus (TBSV). The formation of the TBSV replicase required two purified recombinant TBSV replication proteins, which were obtained from E. coli, the viral RNA replicon, rATP, rGTP, and a yeast cell-free extract. The in vitro assembly of the replicase took place in the membraneous fraction of the yeast extract, in which the viral replicase-RNA complex became RNase- and proteinase-resistant. The assembly of the replicase complex required the heat shock protein 70 (Hsp70 = yeast Ssa1/2p) present in the soluble fraction of the yeast cell-free extract. The assembled TBSV replicase performed a complete replication cycle, synthesizing RNA complementary to the provided RNA replicon and using the complementary RNA as template to synthesize new TBSV replicon RNA.
Keywords: hsp70, RNA-dependent RNA polymerase, viral replicase, virus replication, yeast cell-free extract
Replication of plus-strand (+)RNA viruses is driven by the viral replicase complex in the infected cells. The viral replicase complex assembles on intracellular membrane surfaces from viral replication proteins, the recruited viral RNA and a set of coopted host proteins. This is followed by complementary (−)-strand synthesis and the production of excess amounts of new (+)RNA progeny, which is released from the site of replication to the cytosol (1, 2). Despite its key significance in all RNA virus infections, the composition of the viral replicase complex is not yet fully revealed and the mechanism of assembly is poorly understood (3, 4). Dissection of the functions of the viral and host proteins in the replicase complex would be greatly facilitated by an in vitro approach that leads to the assembly of functional viral replicase complex composed at least partially of defined components.
The major difficulties in achieving in vitro assembly are related to the complex composition of the viral replicase and the requirement for activation of the viral RNA-dependent RNA polymerase (RdRp) during the assembly of the viral replicase in membraneous structures, as evidenced by results with p92pol of Tomato bushy stunt virus (TBSV), 2apol of Brome mosaic virus (BMV), P2 of Alfalfa mosaic virus (AMV), 180K of Tomato mosaic virus (ToMV), and the hepatitis C virus (HCV) NS5B. Most of our knowledge on viral RdRps is based on detergent-solubilized, ribonuclease treated (to remove the endogenous viral RNA), template-dependent viral replicase preparations, which are mostly capable of initiation, elongation, and termination of complementary RNA synthesis or RNA recombination (5–7). These replicase preparations are incapable of performing full replication cycle, show less stringent template specificity than in vivo viral replicases, and use exogenously added RNA templates inefficiently. In addition, cell-free replication assays using extracts obtained from noninfected cells, which are based on coupled translation/replication, have also been used to study viral replicases, including poliovirus, ToMV and TBSV (8–12).
The tombusvirus replicase is among the best-characterized for (+)RNA viruses due to the development of in vitro assays and yeast as a model host (13–17). Replication of tombusvirus RNA depends on two viral-coded factors, namely p92pol RdRp and an auxiliary replication protein, termed p33. These viral proteins in combination with 4–10 host proteins and cellular (e.g., peroxisomal) membranes assemble the viral replicase complex in the yeast model host (18, 19). The list of identified host proteins within the functional replicase includes Ssa1p and Ssa2p (heat shock protein 70, Hsp70), whose down-regulation leads to markedly reduced replication (13). In addition, glyceraldehyde-3-phosphate dehydrogenase (GAPDH, coded by TDH2 and TDH3 genes in yeast) has been shown to bind to the minus-stranded TBSV RNA and regulates plus-strand synthesis (20). Moreover, Cdc34p ubiquitin-conjugating enzyme has been identified as a component of the replicase complex and is involved in ubiquitination of the p33 replication cofactor (21). It is currently not known whether the above host factors might affect the assembly of the viral replicase complex.
In this article, we used purified recombinant TBSV p92pol and p33 replication proteins from a nonplant source in combination with a surrogate viral RNA template to reconstitute the active TBSV replicase in vitro. The in vitro assembly of the functional tombusvirus replicase required rATP and rGTP and the membranous fraction and Ssa1p (Hsp70). We show that the in vitro assembled TBSV replicase can be used to recapitulate many steps of the replication process.
Results
In Vitro Reconstitution of the Functional TBSV Replicase.
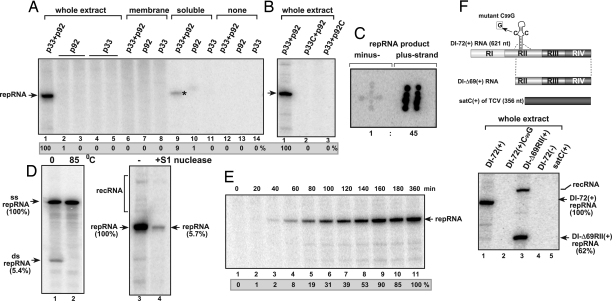
The recombinant TBSV p92pol alone or mixed with recombinant p33 replication cofactor is not a functional RdRp in vitro in the presence of a viral RNA template [termed DI-72(+) repRNA and derived by deletion from the TBSV genomic RNA, Fig. 1F] (Fig. 1A, lanes 12 and 13) (14, 22), suggesting that the host cells might provide additional factors to assemble the functional replicase complex. To test whether assembly of the TBSV replicase complex can be stimulated by components of the host cell, we added purified recombinant TBSV p92pol and p33 replication proteins (both proteins were purified from E. coli), as well as DI-72(+) repRNA to a cell-free extract prepared from untransformed yeast. We observed that the in vitro formed TBSV replicase supported the synthesis of 32P-labeled full-length repRNA in this replicase assay (Fig. 1A, lane 1), suggesting that the viral replicase successfully assembled under the in vitro condition. The control assays lacking either p33 (lanes 2 and 3) or p92pol (lanes 4 and 5) did not give 32P-labeled full-length DI-72 RNA product, excluding the possibility that the yeast cell-free extract contained a terminal transferase or an RNA polymerase that would be responsible for nonspecific labeling of the TBSV repRNA. Similarly, the combination of a defective p33 mutant (i.e., p33C with deletion of the N-terminal half of p33) and the full-length p92pol (Fig. 1B, lane 2) or the full-length p33 and a nonfunctional p92pol mutant (i.e., p92C with deletion of the N-terminal sequence of p92pol that overlaps with p33) (lane 3) did not support TBSV RNA synthesis, confirming previous findings from in vivo experiments that these regions of p33 and p92pol are essential for a functional viral replicase (1). Based on these data, we conclude that a cell-free extract prepared from wild-type (wt) yeast can provide the missing factors for the assembly of the TBSV replicase if recombinant p33 and p92pol are provided.
Fig. 1.
In vitro assembly of the TBSV replicase. (A) Purified recombinant p33 and p92pol replication proteins of TBSV in combination with DI-72 (+)repRNA were added to the cell-free extract (lanes 1–5), to the membrane fraction (lanes 6–8), to the soluble fraction of the yeast cell-free extract (lanes 9–11) or to the buffer only (lanes 12–14). The denaturing PAGE analysis of the 32P-labeled repRNA products obtained is shown. The full-length repRNA is pointed at by an arrow, whereas the small amount of repRNA product, which is likely due to small level of membrane contamination in the soluble fraction, is marked by an asterisk. (B) Testing the effect of mutations in p33 and p92pol on the activity of the reconstituted TBSV replicase. The denaturing PAGE analysis of the replicase products is as shown in Panel A. Note that p33C lacks the N-terminal portion important for membrane binding, whereas p92C lacks the entire N-terminal portion that overlaps with p33. (C) The 32P-labeled RNA products obtained with the in vitro assembled TBSV replicase programmed with DI-72(+) repRNA were used as probes to hybridize with equal amounts of unlabeled DI-72(+) and (−)RNAs, which had been spotted on the membrane in patterns resembling the shape of “+” and “‖”, respectively. The ratio of (+) and (−) RNA products was calculated by using Imagequant software. (D) Detection of single- and double-stranded RNA products produced by the reconstituted TBSV replicase. (Left) The nondenaturing PAGE shows the dsRNA product after phenol-chloroform extraction (lane 1), which can be fully denatured at 85°C (lane 2). (Right)Denaturing PAGE analysis of the products obtained in the in vitro reconstitution assay without treatment (lane 3) or after S1 nuclease treatment (lane 4), which removes the ssRNA, but not the dsRNA product. recRNA represents recombinant RNAs generated from (+)repRNA (27). (E) Time course analysis of the synthesis of repRNA in the in vitro reconstitution assay. The samples were taken at the shown time points. The denaturing PAGE analysis of the products was done as in A. (F) High template specificity of the reconstituted TBSV replicase in vitro during replication of various viral RNAs. (Top) Schematic representation of the RNA constructs used. Note that satC is a heterologous RNA associated with TCV infections. (Bottom) The denaturing PAGE analysis of the 32P-labeled repRNA products obtained is shown. The head-to-tail recRNA is shown as well.
To investigate the polarity of the synthesized viral RNA products by the in vitro formed TBSV replicase, we analyzed the accumulation of plus- versus minus-stranded repRNA products via RNA hybridization using the 32P-labeled repRNA products from the cell-free extract programmed with (+)repRNA and recombinant p33/p92pol as a probe against equal amounts of unlabeled DI-72(+) and (−)RNA transcripts blotted on the membrane (Fig. 1C). This assay revealed that majority of the new repRNA products made by the in vitro assembled replicase is plus-stranded. The (+):(−) ratio was estimated to be 45:1 (Fig. 1C), which is comparable with the ratio obtained with the plant- and yeast-derived membrane-bound tombusvirus replicases (14).
To test further whether the in vitro assembled replicase is capable of a complete cycle of replication using the (+)repRNA as template, we isolated double-stranded (ds) RNA products from the in vitro assays. We found that ≈5% of newly synthesized repRNA products were in dsRNA form after phenol-chloroform extraction (Fig. 1D, lane 1), and these were readily denatured by heat treatment (lane 2). Moreover, 95% of the newly made RNA products were sensitive to S1 single strand-specific RNase (Fig. 1D, lanes 3 and 4), indicating that the majority of the RNA products from the in vitro assay is single-stranded (+)repRNA. These data suggest that a small fraction (2.5–5%) of the newly synthesized RNA is (−)repRNA, whereas the vast majority of new RNA is (+)-stranded, demonstrating that the in vitro formed replicase is capable of a full-cycle of asymmetrical replication on the added (+)repRNA.
Time course experiments revealed that the assembly of the functional replicase took place in 40-to-60 min (Fig. 1E, lanes 3 and 4) and RNA synthesis was the most efficient between 60–160 min (lanes 4–9). Incubations of >3 h did not lead to further RNA synthesis, suggesting that the in vitro incubation mixture accumulates only one (−)repRNA template per replicase complex, which directs synthesis of new (+)repRNA molecules, but the latter do not seem to direct a new cycle of replication.
To characterize the template-specificity of the TBSV replicase complex assembled in vitro, we tested various RNA templates. A single point mutation [mutant DI-72(+)C99G, Fig. 1F, lane 2] in an internal hairpin [termed RII(+)-SL] that interferes with recruitment of the (+)repRNA into replication in vivo (23, 24) and inhibits replicase assembly in vivo (14, 25), also blocked repRNA replication in vitro in the replicase reconstitution assay. This finding supports the idea that RNA recruitment is also required under the in vitro conditions. In addition, the (−)repRNA and the heterologous satC(+) RNA (Fig. 1F) (26), which cannot initiate replication by the TBSV replicase in vivo (1), were not used as templates in our in vitro assay containing the cell-free extract and recombinant p33/p92pol. Testing the highly recombinogenic DI-Δ69RII(+), which lacks the 5′ terminal 238 nt of the 621-nt long DI-72 (27), revealed the emergence of recombinant (head-to-tail dimer-sized) RNAs (Fig. 1F, lane 3). Similar recombinants are also generated in yeast cells and in plant protoplasts as well (27). Our observations of template specificity and production of recombinant RNAs by the in vitro-assembled replicase suggests that in vitro reconstitution in the cell free extract leads to the formation of an authentic TBSV replicase.
Requirement for a Soluble Factor for the in Vitro Assembly of the Functional TBSV Replicase.
Next, we have tested whether the membrane or soluble fractions of the yeast extract were sufficient to facilitate the reconstitution of the TBSV replicase in vitro. The membrane fraction of the yeast extract did not support replication in the presence of purified recombinant TBSV p92pol/p33 and DI-72(+) repRNA (Fig. 1A, lane 6), suggesting that a soluble factor(s) is essential for the assembly of the TBSV replicase. Similar experiment with the soluble fraction of the yeast extract revealed trace amount of replicase product (Fig. 1A, lane 9), which is likely due to contamination of the soluble fraction with small amount of broken membrane fragments. Overall, these experiments indicated that both the soluble and the membrane fractions contain host components that are needed for the in vitro formation of the TBSV replicase.
Requirement for rATP and rGTP During the in Vitro Reconstitution of the TBSV Replicase.
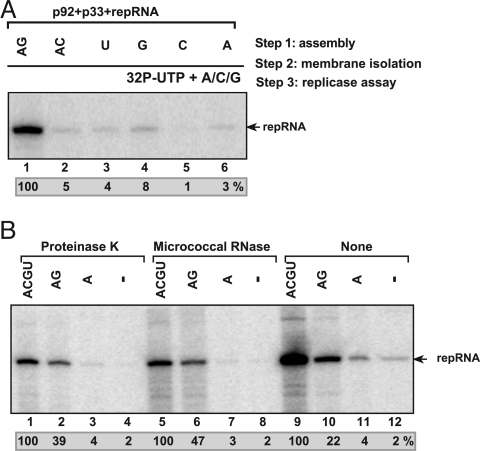
To further test factors affecting the in vitro formation of the TBSV replicase, we developed a stepwise procedure. In step 1, we added the purified p33, p92pol, and DI-72(+) repRNA in combination with a mixture of unlabeled ribonucleotides to the yeast cell-free extract (Fig. 2A). After 1-h incubation (step 2), we collected the membrane fraction of the cell-free extract that likely contained the assembled TBSV replicase complex, while we discarded the soluble fraction containing residual purified p33, p92pol, and DI-72(+) repRNA, which have not yet associated with the membranes. In step 3, we performed a standard replicase assay in the presence of the membrane fraction from step 2 and 32P-labeled UTP and the other ribonucleotides for 3 h. These experiments revealed membrane-bound TBSV replicase formation when all four ribonucleoside triphosphates (Fig. 2B, lane 9) or combination of rATP and rGTP (Fig. 2A, lane 1) were supplied at step 1, based on the appearance of activity at step 3. We observed only low level of replicase activity when rUTP, rGTP, rCTP, or rATP were supplied separately or in combination (rATP/rCTP) at step 1 (Fig. 2A, lanes 2–6). Altogether, the assembly of the viral replicase does not seem to require minus-strand synthesis (which would occur only if all four rNTPs are present), but the assembly seems to require rATP and rGTP to stimulate a currently unknown process.
Fig. 2.
Critical role for ribonucleotides during the reconstitution of the TBSV replicase. (A) A stepwise approach was used to separate the possible role of ribonucleotides during the assembly of the TBSV replicase and during RNA synthesis. In step 1, the purified recombinant TBSV p33, p92pol and (+)repRNA were added to the cell-free extract in the presence of various unlabeled ribonucleotides as shown. This was followed by removal of the extra amount of p33, p92pol and repRNA, which were not bound to the membranes of cell-free extract, and then by the standard replicase assay in a buffer containing 32P-UTP and ATP, CTP, and GTP (step 3). The denaturing PAGE analysis of the 32P-labeled repRNA products obtained is shown. (B) The in vitro assembled TBSV replicase forms a protease/ribonuclease-resistant structure in the yeast cell-free extract. The in vitro reconstitution in the presence of various ribonucleotides was done in three steps as described in A. Note that we applied a 15-min treatment with either proteinase K or ribonuclease (micrococcal nuclease) at the end of step 1, before centrifugation (step 2), which removed the proteinase K and the ribonuclease as well as the extra amount of p33, p92pol, and repRNA not bound to the membranes in the cell-free extract. The denaturing PAGE analysis of the 32P-labeled repRNA products obtained is shown.
Because replication of TBSV takes place in spherules formed in membranous compartment of the peroxisome (28), we wanted to test whether the reconstituted replicase complex after step 1 becomes protected from RNases or proteases. After performing step 1 for 1 h in the presence of purified p33, p92pol, and DI-72(+) repRNA and various combination of ribonucleotides in the yeast cell-free extract, we treated the extract with micrococcal nuclease or proteinase K for 15 min, followed by removal of these reagents in step 2 (Fig. 2B). Then, we performed the replicase assay (step 3) in the presence of 32P-labeled UTP and the other ribonucleotides for 3 h. These experiments revealed that the replicase assembled in vitro in step 1 was protected from micrococcal nuclease and proteinase K if the assembly took place in the presence of A/C/G/U or A/G ribonucleotides (Fig. 2B, lanes 1, 2, 5, and 6). These data suggest that the TBSV replicase can assemble in vitro in the membranous compartment into an RNase/protease-insensitive complex in the absence of minus-strand synthesis.
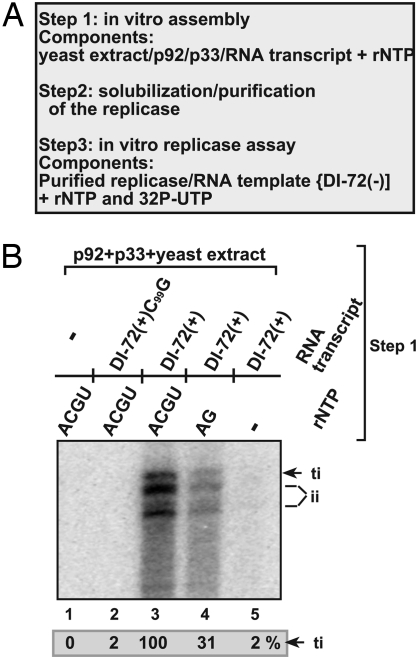
To test whether the in vitro formation of the TBSV replicase leads to a stable complex, similar to the situation when the assembly takes place in vivo, we have affinity purified the TBSV replicase complex from the yeast cell-free extract after nonionic detergent-based solubilization (Fig. 3A) (14, 25). Because of the loss of the endogenous RNA template during the affinity purification, we have tested the solubilized and affinity purified replicase with added DI-72(−) repRNA template. We used (−) polarity rather than (+) polarity template to prevent a possible activation of the solubilized replicase, and because DI-72 (−)RNA is an efficient template for the solubilized/purified replicase in vitro (14). We found that the solubilized/purified TBSV replicase preparation was active only when purified from the yeast cell-free extract containing p33, p92pol, and DI-72(+) repRNA and rATP and rGTP (Fig. 3B, lanes 3 and 4). The obtained 32P-labeled RNA products included the correct 3′ terminal and internal initiation products as well, suggesting that the solubilized/purified replicase from the reconstitution assay lost its precision during initiation as observed with solubilized replicases from plants and yeast (14). Importantly, the purified replicase was not active when obtained from the reconstitution assay lacking (+)repRNA (Fig. 3B, lane 1), suggesting that, similar to the in vivo situation (14, 25), the viral (+)repRNA was required for obtaining active purified TBSV replicase complex (Fig. 3B, lane 3). Moreover, mutant DI-72(+)C99G, which is deficient in binding to p33 and in recruitment for replication, did not support the assembly of an active TBSV replicase in the reconstitution assay (Fig. 3B, lane 2), confirming previous in vivo findings (14, 25). Based on these data, we conclude that the TBSV replicase purified from the in vitro reconstitution assay maintains its activity after solubilization/purification with exogenous RNA templates, but shows reduced precision during initiation.
Fig. 3.
Purification and characterization of the in vitro formed TBSV replicase. (A) A schematic presentation of the in vitro assay. The reconstitution assay contained the cell-free extract, the MBP-affinity purified recombinant TBSV p33 and p92pol, various (+)repRNAs as well as various unlabeled ribonucleotides as shown in B. After 1-hour reconstitution, the membrane-bound replicase was solubilized with Triton X-100/SB3–10 detergent, followed by purification on Ni-column of the 6xHis/MBP-tagged p33, which is integral part of the replicase complex. The activity of the affinity-purified TBSV replicase was tested on DI-72(−) RNA added to each sample using the same amount of RNA. (B) The denaturing PAGE analysis of the 32P-labeled DI-72(−) RNA products obtained with the solubilized and purified TBSV replicase is shown. Note that the purified, template-dependent TBSV replicase initiates RNA synthesis precisely from the 3′ end (the product labeled as ti) as well as imprecisely at internal positions on DI-72(−) RNA (indicated as ii products).
Hsp70 Proteins Ssa1p and Ssa2p Are Required for the In Vitro Reconstitution of the TBSV Replicase.
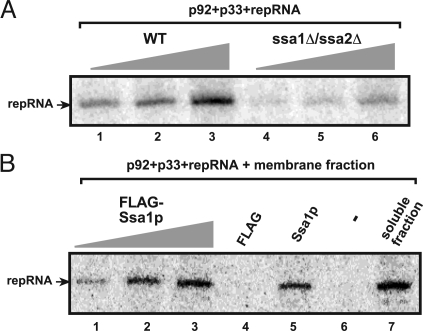
Because the above experiments indicated that rATP/rGTP as well as a soluble (i.e., cytosolic) host factor are required for the in vitro formation of the TBSV replicase, we predicted that Ssa1/2p Hsp70 could be the required cytosolic host factor. The prediction was based on our earlier finding that Ssa1/2p were part of the highly purified tombusvirus replicase complex (13). To test this idea, we have prepared the cell-free extract from a yeast strain (ssa1 ssa2) lacking functional, constitutively expressed Hsp70 genes (29). The in vitro reconstitution assay based on the cell-free extract from ssa1 ssa2 strain resulted in a TBSV replicase with diminished activity (≈22%) when compared with the cell-free extract prepared from wt yeast (Fig. 4A, compare lanes 3 and 6). This finding supported the involvement of Ssa1/2p in the in vitro assembly of the TBSV replicase.
Fig. 4.
Ssa1p is required for the in vitro assembly of the TBSV replicase. (A) The purified recombinant TBSV p33/p92pol and (+)repRNA were added to the cell-free extract prepared from untransformed (TBSV-free) wt or mutated yeast (ssa1ssa2), which were used in increasing amounts. The denaturing PAGE analysis of the 32P-labeled repRNA products obtained in the in vitro reconstitution assay is shown. (B) Purified recombinant Ssa1p stimulates the assembly of the TBSV replicase in vitro. The membrane fraction of the cell-free extract prepared from untransformed (TBSV-free) wt yeast was programmed with purified recombinant TBSV p33/p92pol and (+)repRNA. The samples also contained 0.2, 1, and 2 μg of Ssa1p (lanes 1–3), FLAG peptide, buffer only (lane 6) or soluble fraction of the cell-free extract as a positive control (lane 7). The 32P-labeled repRNA products obtained in the in vitro reconstitution assay were analyzed with denaturing PAGE.
To obtain more direct evidence for the involvement of Ssa1/2p in the replicase assembly, we used the membrane fraction of the cell-free extract from wt yeast, which is incompetent in the vitro reconstitution assay (Fig. 1A, lane 6). However, the addition of FLAG-affinity purified Ssa1p to the reconstitution assay with the membrane fraction of the cell-free extract resulted in the assembly of the functional TBSV replicase in vitro (Fig. 4B, lane 5), which was as active as the replicase assembled in the presence of the combined membrane + soluble fractions (lane 7). Moreover, the relative activity of the TBSV replicase assembled in the membrane fraction correlated with the amount of Ssa1p added to the assay (Fig. 4B, lanes 1–3).
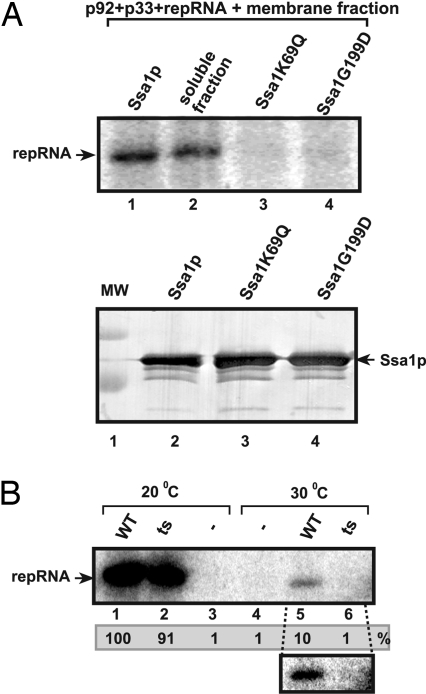
Even more convincing evidence on the role of Ssa1p in the reconstitution of the active TBSV replicase was obtained by using loss-of-function Ssa1p mutants. Indeed, two Ssa1p mutants deficient in ATP hydrolysis (30) could not facilitate the assembly of the TBSV replicase in the vitro reconstitution assay (Fig. 5A, lanes 3 and 4), suggesting that functional Ssa1p is required to assist the assembly of the TBSV replicase. These data in combination with the negative results obtained with the FLAG peptide (Fig. 4B, lane 4), which was purified from yeast using the same method used for Ssa1p, excludes the possibility that a nonspecific contaminating protein(s) was responsible in assisting the assembly of the TBSV replicase in vitro.
Fig. 5.
Loss-of function Ssa1p mutants cannot stimulate the in vitro assembly of the TBSV replicase. (A) The membrane fraction of the cell-free extract prepared from untransformed (TBSV-free) wt yeast was programmed with purified recombinant TBSV p33 and p92pol and (+)repRNA. The samples also contained purified wt Ssa1p (lane 1), the soluble fraction of the cell-free extract (lane 2), and two loss-of function Ssa1p mutants deficient in ATP hydrolysis (lanes 3 and 4). The 32P-labeled repRNA products obtained in the in vitro reconstitution assay were analyzed with denaturing PAGE. (Bottom) Western blot analysis of affinity-purified Ssa1p and mutants used in the reconstitution assay. (B) Lack of stimulation of the in vitro assembly of the TBSV replicase by a ts mutant of Ssa1p at a nonpermissive temperature. The 32P-labeled repRNA products obtained in the in vitro reconstitution assay were analyzed with denaturing PAGE. The purified wt and ts Ssa1p facilitated the in vitro assembly of the TBSV replicase at 20°C (lanes 1 and 2), whereas only the wt Ssa1p promoted the assembly process at 30°C (lane 5), and the ts mutant was inactive (lane 6). A subset of the panel shows the longer exposure of the portion of the gel.
Another approach to demonstrate the critical role of ts Ssa1p in TBSV replicase assembly in vitro was based on a temperature-sensitive (ts) mutant. The FLAG-affinity purified tsSSA1p was almost as efficient as the wt Ssa1p in assisting TBSV replicase assembly in the reconstitution assay at 20°C (Fig. 5B, lanes 1 and 2), whereas the ts Ssa1p did not assist the assembly at 30°C at all (lanes 5 and 6). Based on the above data with loss-of-function Ssa1p mutants, we conclude that Ssa1p Hsp70 protein is required for the assembly of the TBSV replicase.
Discussion
In vitro assembly of the TBSV replicase requires viral and host proteins, rATP/rGTP as well as cellular membranes. We have successfully assembled in vitro a plus-strand RNA virus replicase, namely the TBSV replicase, using purified recombinant viral proteins expressed in E. coli, viral RNA transcript, ribonucleotides and a yeast cell-free extract (Fig. 1). Whereas the full-length TBSV p33 and p92pol replication proteins were functional together, p92pol was an inactive RdRp in the absence of p33 in the reconstitution assay. Also, truncated versions of p33 and p92pol were not functional, similar to their behavior in yeast cells. These findings confirm that both p33 and p92pol are essential replication proteins. Moreover, the in vitro assembled TBSV replicase seems to recapitulate the features of the in vivo viral replicase.
In addition to the viral replication proteins, the in vitro formation of the TBSV replicase depended on other factors as well. As expected, cellular membranes, which are also part of viral replicases forming spherules or vesicles in infected cells (3, 31), were also essential for the formation of the functional replicase. The membrane is not only important structurally by giving a boundary to the replicase complex, but also functionally during viral RNA synthesis. Accordingly, removal of the membrane structure by solubilization with nonionic detergents and purification results in replicases that have different features from the membrane-bound replicases, including reduced template specificity, ability to synthesize only the complementary RNA, efficient 3′-terminal extension, and high frequency of template switching (recombination) (5–7, 19). Altogether, the TBSV replicase assembled in vitro shows remarkably similar features with the in vivo replicase, much more so than the widely used solubilized/purified replicase.
Cytosolic Hsp70 Is Required for the Reconstitution of the TBSV Replicase.
The in vitro formation of the TBSV replicase also required the cytosolic (soluble) fraction of the yeast cell-free extract, suggesting that a host protein(s) might be present in this fraction. Accordingly, we have found that Ssa1p Hsp70 protein chaperone was required for the reconstitution of the TBSV replicase. Ssa1p/Ssa2p is part of the tombusvirus replicase complex and it is required for TBSV replication in yeast (13) and in plant host, too (R.Y.-L. Wang, J.S., and P.D.N., unpublished work). Based on these observations, we propose that Hsp70 assists the assembly of the TBSV replicase both in vivo and in vitro. The rATP-hydrolysis function of Ssa1p is required for the assembly, suggesting that Ssa1p is likely involved in refolding the components of the viral replicase during the in vitro reconstitution. Accordingly, rATP has to be added to the in vitro reconstitution assay, likely to stimulate the activity of Ssa1p, which is driven by rATP hydrolysis (32). We have also found that rGTP is required for efficient assembly, although we do not know yet the significance of rGTP during the assembly steps. It is unlikely that rATP/rGTP are required for the initiation of minus-strand synthesis, because minus-strand synthesis starts with 5′-GGGCU. (33), so only the first three nucleotides of the minus-strand could be synthesized by the replicase in the presence of rATP/rGTP.
The heat shock chaperone family, including Hsp70, the J-domain group, and Hsp90, is a major group of host factors implicated in replication of plus-stranded (e.g., HCV), minus-stranded RNA viruses (influenza and vesicular stomatitis virus), retroviruses (HIV), hepatitis B virus and other RNA viruses (34–42). The polymerase of respiratory syncytial virus (RSV) has been shown to colocalize with Hsp70 to lipid-raft membranes and virus-induced inclusion bodies (34). Moreover, additional members of the heat shock protein family were shown to be important for stimulation of polymerase activity of Influenza virus (37), enhancement of Flock house virus and BMV replication (43), activation of reverse transcriptase for hepadnaviruses (44), or involved in the assembly of closterovirus virions (45). The induction of robust expression of subset of Hsp70 genes by various plant viruses has been observed, suggesting that cytosolic Hsp70 proteins could affect stability/function of viral proteins during infections (46, 47). Therefore, Hsp70 and cofactors might play roles in assembly of viral replicases as shown for TBSV in this work.
Remarkable Features of the in Vitro Assembled TBSV Replicase.
The in vitro reconstituted TBSV replicase not only shows the advantages of the solubilized/purified replicases, such as de novo initiation of RNA synthesis, recombination and so on, it also has several remarkable features. These include high template specificity, which leads to the in vitro replication of the TBSV (+)RNA, but not mutated or heterologous viral RNAs, which are also defective in replication in vivo. The in vitro formed TBSV replicase is also capable of full RNA replication, including asymmetrical RNA synthesis, which leads to the production of approximately 30–40-fold more (+)- then (−)RNAs (Fig. 1D). Moreover, the in vitro assembled TBSV replicase and its RNA content is remarkably resistant against single-strand specific ribonuclease and a protease, suggesting that the membranes present in the yeast extract can facilitate the formation of protected replicase structures, unlike the solubilized/purified replicases.
Conclusion.
The efficient in vitro formation of the TBSV replicase makes it suitable to study viral and host factors affecting the assembly of the replicase, the nature of high template specificity, and the asymmetrical, complete cycle of replication. This progress allows to gain more insights into the mechanistic features of replicase complexes of (+)RNA viruses, which are primary targets for antiviral approaches.
Materials and Methods
Preparation of the Cell-Free Yeast Extract.
Cell-free yeast extract was prepared according to (12), except that untransformed BY4741 yeast grown in YPD media was used for the preparation of the extract.
Replication Assay By Using the Cell-Free Extract.
The replication assay was done according to (12), except that the reaction mix contained 50 mM potassium acetate and 0.5 μg recombinant TBSV MBP-p33 and/or TBSV MBP-p92. For fractionation of the cell-free extract, the extract was centrifuged at 4°C for 10 min to separate the “soluble” (supernatant) and “membrane” (pellet) fraction (21,000 × g). To remove possible contaminating soluble proteins, the pellet was washed with buffer A (30 mM Hepes-KOH pH 7.4, 100 mM potassium acetate, and 2 mM magnesium acetate) followed by centrifugation at 4°C for 10 min and resuspension of the pellet in buffer A. Nickel-affinity purification of the 6xHis/MBP-p33 from the in vitro extract and testing of TBSV RdRp activity using an exogenous template [DI-72(−)] was done by using methods described earlier (14).
Proteinase K and Micrococcal Nuclease Treatment.
The replication assay was conducted as described above, except that only the indicated nucleotides were added and the 32P -UTP was omitted during the first 1h of the incubation at 25°C. Then the samples were either treated with Proteinase K (0.05 mg/ml, final concentration) or micrococcal nuclease (0.04 U/ml, final concentration, and 1 mM CaCl2), or left untreated for 15 min at 25°C. To terminate the reaction, we added 2.5 mM EGTA to the samples treated with micrococcal nuclease and all reaction mixtures with micrococcal nuclease or proteinase K were centrifuged at 4°C for 5 min. The supernatant was discarded and the pellet was washed with buffer A, and then centrifuged for 5 min at 4°C. The pellet was dissolved in 5 μl of buffer A and 4 μl of 4× reaction buffer, creatine kinase, creatine phosphate, DTT, actinomycin D, RNase inhibitor, 1 mM ATP, CTP, GTP, and 0.02 mM UTP, and 32P-UTP were added according to ref. 12 in a 20-μl reaction. The reaction mix was incubated for three h at 25°C. S1 digestion of the in vitro replicase products and dsRNA detection was according to ref. 12.
Expression and Purification of Wild-Type and Mutant Ssa1p from Yeast.
The copper-inducible CUP1 promoter was used to express the wt FLAG-tagged Ssa1p and the temperature sensitive (ts) Ssa1p (49) [kindly provided by Elizabeth Craig (University of Wisconsin, Madison)] from plasmid pEsc-His/Cup-FLAG/ssa1 wt and pEsc-His/Cup-FLAG/ssa1ts. The expression of recombinant proteins was induced by the addition of 50 μM copper-sulfate for 3 h at 30°C (50). The proteins were purified according to (27), except that the Fastprep machine (done twice for 20 sec at 4 m/s) was used to break the cells in buffer A (12). The recombinant proteins were eluted from the Anti-FLAG M2 resin by using 100 μg/ml FLAG peptide in buffer A. The purified proteins were stained with Coomassie Brilliant Blue following SDS/PAGE. We have tested 0.2–2 μg of purified proteins added to the in vitro reaction.
Acknowledgments.
This work was supported by National Science Foundation Grant MCB0078152, National Institutes of Health–National Institute of Allergy and Infectious Diseases, and University of Kentucky (P.D.N.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Panavas T, Hawkins CM, Panaviene Z, Nagy PD. The role of the p33:p33/p92 interaction domain in RNA replication and intracellular localization of p33 and p92 proteins of Cucumber necrosis tombusvirus. Virology. 2005;338:81–95. doi: 10.1016/j.virol.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Ahlquist P, Noueiry AO, Lee WM, Kushner DB, Dye BT. Host factors in positive-strand RNA virus genome replication. J Virol. 2003;77:8181–88186. doi: 10.1128/JVI.77.15.8181-8186.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salonen A, Ahola T, Kaariainen L. Viral RNA replication in association with cellular membranes. Curr Top Microbiol Immunol. 2005;285:139–173. doi: 10.1007/3-540-26764-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagy PD, Pogany J. Multiple roles of viral replication proteins in plant RNA virus replication. Methods Mol Biol. 2008;451:55–68. doi: 10.1007/978-1-59745-102-4_4. [DOI] [PubMed] [Google Scholar]
- 5.Kao CC, Singh P, Ecker DJ. De novo initiation of viral RNA-dependent RNA synthesis. Virology. 2001;287:251–260. doi: 10.1006/viro.2001.1039. [DOI] [PubMed] [Google Scholar]
- 6.Kim MJ, Kao C. Factors regulating template switch in vitro by viral RNA-dependent RNA polymerases: Implications for RNA-RNA recombination. Proc Natl Acad Sci USA. 2001;98:4972–4977. doi: 10.1073/pnas.081077198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng CP, Nagy PD. Mechanism of RNA recombination in carmo- and tombusviruses: Evidence for template switching by the RNA-dependent RNA polymerase in vitro. J Virol. 2003;77:12033–12047. doi: 10.1128/JVI.77.22.12033-12047.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barton DJ, Flanegan JB. Coupled translation and replication of poliovirus RNA in vitro: Synthesis of functional 3D polymerase and infectious virus. J Virol. 1993;67:822–831. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duggal R, Cuconati A, Gromeier M, Wimmer E. Genetic recombination of poliovirus in a cell-free system. Proc Natl Acad Sci USA. 1997;94:13786–13791. doi: 10.1073/pnas.94.25.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komoda K, Naito S, Ishikawa M. Replication of plant RNA virus genomes in a cell-free extract of evacuolated plant protoplasts. Proc Natl Acad Sci USA. 2004;101:1863–1867. doi: 10.1073/pnas.0307131101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang RS, Barton DJ, Flanegan JB, Kirkegaard K. Poliovirus RNA recombination in cell-free extracts. RNA. 1997;3:624–633. [PMC free article] [PubMed] [Google Scholar]
- 12.Pogany J, Nagy PD. Authentic replication and recombination of Tomato bushy stunt virus RNA in a cell-free extract from yeast. J Virol. 2008;82:5967–5980. doi: 10.1128/JVI.02737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serva S, Nagy PD. Proteomics analysis of the tombusvirus replicase: Hsp70 molecular chaperone is associated with the replicase and enhances viral RNA replication. J Virol. 2006;80:2162–2169. doi: 10.1128/JVI.80.5.2162-2169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panaviene Z, Panavas T, Serva S, Nagy PD. Purification of the cucumber necrosis virus replicase from yeast cells: Role of coexpressed viral RNA in stimulation of replicase activity. J Virol. 2004;78:8254–8263. doi: 10.1128/JVI.78.15.8254-8263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panavas T, Nagy PD. Yeast as a model host to study replication and recombination of defective interfering RNA of Tomato bushy stunt virus. Virology. 2003;314:315–325. doi: 10.1016/s0042-6822(03)00436-7. [DOI] [PubMed] [Google Scholar]
- 16.Nagy PD, Pogany J. Partial purification and characterization of Cucumber necrosis virus and Tomato bushy stunt virus RNA-dependent RNA polymerases: Similarities and differences in template usage between tombusvirus and carmovirus RNA-dependent RNA polymerases. Virology. 2000;276:279–288. doi: 10.1006/viro.2000.0577. [DOI] [PubMed] [Google Scholar]
- 17.Nagy PD. Yeast as a model host to explore plant virus-host interactions. Annu Rev Phytopathol. 2008;46:217–242. doi: 10.1146/annurev.phyto.121407.093958. [DOI] [PubMed] [Google Scholar]
- 18.Nagy PD, Pogany J. Yeast as a model host to dissect functions of viral and host factors in tombusvirus replication. Virology. 2006;344:211–220. doi: 10.1016/j.virol.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 19.White KA, Nagy PD. Advances in the molecular biology of tombusviruses: Gene expression, genome replication, and recombination. Prog Nucleic Acid Res Mol Biol. 2004;78:187–226. doi: 10.1016/S0079-6603(04)78005-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang RY, Nagy PD. Tomato bushy stunt virus co-opts the RNA-binding function of a host metabolic enzyme for viral genomic RNA synthesis. Cell Host Microbe. 2008;3:178–187. doi: 10.1016/j.chom.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Barajas D, Panavas T, Herbst DA, Nagy PD. Cdc34p ubiquitin-conjugating enzyme is a component of the tombusvirus replicase complex and ubiquitinates p33 replication protein. J Virol. 2008;82:6911–6926. doi: 10.1128/JVI.00702-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajendran KS, Nagy PD. Interaction between the replicase proteins of Tomato bushy stunt virus in vitro and in vivo. Virology. 2004;326:250–261. doi: 10.1016/j.virol.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Pogany J, White KA, Nagy PD. Specific binding of tombusvirus replication protein p33 to an internal replication element in the viral RNA is essential for replication. J Virol. 2005;79:4859–4869. doi: 10.1128/JVI.79.8.4859-4869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monkewich S, et al. The p92 polymerase coding region contains an internal RNA element required at an early step in tombusvirus genome replication. J Virol. 2005;79:4848–4858. doi: 10.1128/JVI.79.8.4848-4858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panaviene Z, Panavas T, Nagy PD. Role of an internal and two 3′-terminal RNA elements in assembly of tombusvirus replicase. J Virol. 2005;79:10608–10618. doi: 10.1128/JVI.79.16.10608-10618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy PD, Pogany J, Simon AE. RNA elements required for RNA recombination function as replication enhancers in vitro and in vivo in a plus-strand RNA virus. EMBO J. 1999;18:5653–5665. doi: 10.1093/emboj/18.20.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng CP, Serviene E, Nagy PD. Suppression of viral RNA recombination by a host exoribonuclease. J Virol. 2006;80:2631–2640. doi: 10.1128/JVI.80.6.2631-2640.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCartney AW, Greenwood JS, Fabian MR, White KA, Mullen RT. Localization of the Tomato Bushy Stunt Virus Replication Protein p33 Reveals a Peroxisome-to-Endoplasmic Reticulum Sorting Pathway. Plant Cell. 2005;17:3513–3531. doi: 10.1105/tpc.105.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wegele H, Haslbeck M, Buchner J. Recombinant expression and purification of Ssa1p (Hsp70) from Saccharomyces cerevisiae using Pichia pastoris. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;786:109–115. doi: 10.1016/s1570-0232(02)00724-9. [DOI] [PubMed] [Google Scholar]
- 30.McClellan AJ, Brodsky JL. Mutation of the ATP-binding pocket of SSA1 indicates that a functional interaction between Ssa1p and Ydj1p is required for post-translational translocation into the yeast endoplasmic reticulum. Genetics. 2000;156:501–512. doi: 10.1093/genetics/156.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller S, Krijnse-Locker J. Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol. 2008;6:363–374. doi: 10.1038/nrmicro1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saibil HR. Chaperone machines in action. Curr Opin Struct Biol. 2008;18:35–42. doi: 10.1016/j.sbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Pogany J, Fabian MR, White KA, Nagy PD. A replication silencer element in a plus-strand RNA virus. EMBO J. 2003;22:5602–5611. doi: 10.1093/emboj/cdg523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown G, et al. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology. 2005;338:69–80. doi: 10.1016/j.virol.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Castorena KM, Weeks SA, Stapleford KA, Cadwallader AM, Miller DJ. A functional heat shock protein 90 chaperone is essential for efficient flock house virus RNA polymerase synthesis in drosophila cells. J Virol. 2007;81:8412–8420. doi: 10.1128/JVI.00189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar M, Mitra D. Heat shock protein 40 is necessary for human immunodeficiency virus-1 Nef-mediated enhancement of viral gene expression and replication. J Biol Chem. 2005;280:40041–40050. doi: 10.1074/jbc.M508904200. [DOI] [PubMed] [Google Scholar]
- 37.Momose F, Naito T, Yano K, Sugimoto S, Morikawa Y, Nagata K. Identification of Hsp90 as a stimulatory host factor involved in influenza virus RNA synthesis. J Biol Chem. 2002;277:45306–45314. doi: 10.1074/jbc.M206822200. [DOI] [PubMed] [Google Scholar]
- 38.Naito T, Momose F, Kawaguchi A, Nagata K. Involvement of Hsp90 in assembly and nuclear import of influenza virus RNA polymerase subunits. J Virol. 2007;81:1339–1349. doi: 10.1128/JVI.01917-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa S, Umehara T, Matsuda C, Kuge S, Sudoh M, Kohara M. Hsp90 inhibitors suppress HCV replication in replicon cells and humanized liver mice. Biochem Biophys Res Commun. 2007;353:882–888. doi: 10.1016/j.bbrc.2006.12.117. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto T, et al. Hepatitis C virus RNA replication is regulated by FKBP8 and Hsp90. EMBO J. 2006;25:5015–5025. doi: 10.1038/sj.emboj.7601367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sohn SY, Kim SB, Kim J, Ahn BY. Negative regulation of hepatitis B virus replication by cellular Hsp40/DnaJ proteins through destabilization of viral core and X proteins. J Gen Virol. 2006;87:1883–1891. doi: 10.1099/vir.0.81684-0. [DOI] [PubMed] [Google Scholar]
- 42.Tomita Y, Mizuno T, Diez J, Naito S, Ahlquist P, Ishikawa M. Mutation of host DnaJ homolog inhibits brome mosaic virus negative-strand RNA synthesis. J Virol. 2003;77:2990–2997. doi: 10.1128/JVI.77.5.2990-2997.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kampmueller KM, Miller DJ. The cellular chaperone heat shock protein 90 facilitates Flock House virus RNA replication in Drosophila cells. J Virol. 2005;79:6827–6837. doi: 10.1128/JVI.79.11.6827-6837.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu J, Flores D, Toft D, Wang X, Nguyen D. Requirement of heat shock protein 90 for human hepatitis B virus reverse transcriptase function. J Virol. 2004;78:13122–13131. doi: 10.1128/JVI.78.23.13122-13131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alzhanova DV, Napuli AJ, Creamer R, Dolja VV. Cell-to-cell movement and assembly of a plant closterovirus: Roles for the capsid proteins and Hsp70 homolog. EMBO J. 2001;20:6997–7007. doi: 10.1093/emboj/20.24.6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aranda MA, Escaler M, Wang D, Maule AJ. Induction of HSP70 and polyubiquitin expression associated with plant virus replication. Proc Natl Acad Sci USA. 1996;93:15289–15293. doi: 10.1073/pnas.93.26.15289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitham SA, Yang C, Goodin MM. Global impact: Elucidating plant responses to viral infection. Mol Plant Microbe Interact. 2006;19:1207–1215. doi: 10.1094/MPMI-19-1207. [DOI] [PubMed] [Google Scholar]
- 48.Rajendran KS, Nagy PD. Characterization of the RNA-binding domains in the replicase proteins of tomato bushy stunt virus. J Virol. 2003;77:9244–9258. doi: 10.1128/JVI.77.17.9244-9258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becker J, Walter W, Yan W, Craig EA. Functional interaction of cytosolic hsp70 and a DnaJ-related protein, Ydj1p, in protein translocation in vivo. Mol Cell Biol. 1996;16:4378–4386. doi: 10.1128/mcb.16.8.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaag HM, Stork J, Nagy PD. Host transcription factor Rpb11p affects tombusvirus replication and recombination via regulating the accumulation of viral replication proteins. Virology. 2007;368:388–404. doi: 10.1016/j.virol.2007.07.003. [DOI] [PubMed] [Google Scholar]