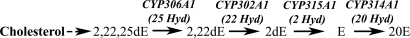Abstract
In female insects, the steroid hormone 20-hydroxyecdysone (20E) plays a major role in activating vitellogenesis, a process required for egg development. By contrast with vertebrates, production of large amounts of hormonal steroids has not been reported in adult male insects. In the present study, we analyzed steroidogenesis in both male and female adult of the malaria mosquito Anopheles gambiae and we found that A. gambiae male mosquitoes produce high amounts of the steroid hormone 20E. Importantly, we found that male accessory glands, but not testes, are the source of 20E. Moreover, this steroid hormone is stored in male accessory glands and delivered to females during mating. These findings suggest that male 20E may not act as a true male sex steroid, but more likely as an allohormone. Our results give new insights into species-specific physiological processes that govern the reproductive success of the malaria mosquito. This could thus lead to the identification of new target genes for manipulating male and/or female reproductive success, a promising way to reduce or eliminate mosquito population and therefore to control malaria transmission.
Keywords: allohormone, ecdysteroids, malaria, male accessory glands, mosquito
The mosquito Anopheles gambiae is the main African vector of Plasmodium falciparum, responsible for the severe forms of human malaria, causing more than 1 million deaths per year (1). At the present time, there is no satisfactory way to completely block malaria transmission, and new approaches are needed (2). To control the transmission of malaria and other vector-borne diseases, diverse new control strategies are being developed for the reduction or elimination of vector populations (3–6). Toward this aim, the sterile male insect technique provides a safe and efficient strategy that limits the growth of insect pest populations (7). Males, produced in a factory and sterilized, are released into the field to reduce the size of wild populations by mating with virgin females, which will then lay unfertilized eggs. In mosquitoes, and more generally in insects, male fertility depends not only on fertile spermatozoa, but also upon secretions transferred to the female during mating to enhance her reproductive success (8, 9). A better knowledge of the reproductive biology of A. gambiae, including hormonal regulation of reproduction, would be beneficial, as this could lead to the identification of new target genes for manipulating male and/or female reproductive success (4, 5, 10).
In insects, specific steroid hormones, called ecdysteroids, play a major role during female reproduction (11–13). In mosquitoes, a blood meal triggers the ovaries to secrete ecdysone (E), subsequently hydroxylated to 20-hydroxyecdysone (20E), which in turn activates the transcription of the vitellogenin (Vg) gene in the female fat body. This leads to the production and secretion of Vg proteins into the hemolymph, before they are incorporated into the growing oocytes (11–13). In male insects, the possible production of steroids and whether these could affect reproductive behavior and fitness have not been well documented, in contrast to vertebrates. To date, ecdysteroid production in males has been reported for a few insect species only, mainly lepidopterans (14, 15). In these cases, steroids were released by testicular follicular sheaths at the end of the last larval instar and during pupal development. Synthesis of steroids has also been observed in several tissues of adult male migratory grasshoppers (order Orthoptera) and in the testes of the adult male blowfly Calliphora vicina (order Diptera) (16, 17). However, only small amounts of steroids were released in vitro when compared with the amounts produced by ovaries, and no prominent compound has been identified. The demonstration of a significant production of one major hormonal steroid is still lacking in male insects, and this has led to the current dogma that they do not have sex steroids (18–20).
The recent identification of genes encoding cytochrome P450 enzymes (CYPs) catalyzing the four terminal steps of ecdysteroidogenesis in Drosophila melanogaster (21–25) (Fig. 1) provided the opportunity to investigate steroidogenesis in insect species of health or economic interest (21, 22, 26–28). In the present study, we analyzed steroidogenesis in male and female A. gambiae during reproductive processes. We found that male accessory glands (MAGs) synthesize high amounts of 20E. Importantly, 20E is mainly stored in MAGs and remarkable amounts of this steroid hormone are transferred to females during mating, strongly suggesting that 20E may not act as a male sex steroid, but may act more as an allohormone by possibly being a modulator of post-mating effects.
Fig. 1.
Biosynthetic pathway of 20E from cholesterol emphasizing the four terminal steps of steroidogenesis catalyzed by CYP306A1, CYP302A1, CYP315A1, and CYP314A1 (25-, 22-, 2-, and 20-hydroxylases, respectively). (2,22,25dE, 2,22,25-trideoxyecdysone; 2,22dE, 2,22-dideoxyecdysone; 2dE, 2-deoxyecdysone.)
Results
Conservation of Steroidogenic CYPs in A. gambiae.
CYP306A1, CYP302A1, CYP315A1, and CYP314A1 catalyze the four final steps of ecdysteroidogenesis in D. melanogaster (Fig. 1). To establish the functional conservation of these CYPs in A. gambiae, we cloned each one of the A. gambiae orthologues into an expression vector, allowing transient expression of the corresponding proteins in transfected SL2 cells. Conversion of radio-labeled substrates by transfected cells was then analyzed by RP-HPLC [see supporting information (SI) Fig. S1 for detailed methods and results]. These experiments demonstrate that AgCYP302A1, AgCYP315A1, and AgCYP314A1 encode the functional 22-, 2- and 20-hydroxylases respectively, as do their Drosophila orthologues (24, 25). Because no radiolabeled substrate was available, the functional conservation of AgCYP306A1, encoding the putative 25-hydroxylase, could not be assessed. However, based on the conservation of the three other steroidogenic CYPs, it is highly probable that CYP306A1 is also functionally conserved in A. gambiae.
Blood-Feeding Triggers Ovarian Ecdysteroid Production.
In anautogenous female mosquitoes, egg production is a cyclic process, named the gonotrophic cycle, which starts with the ingestion of a blood meal, is followed by the synchronous maturation of a set of oocytes, and ends with oviposition (29). This process usually lasts 50 to 72 h at 25 °C in A. gambiae. We analyzed the expression of the four CYPs by RT-PCR in the ovary during the first gonotrophic cycle and in other tissues of 24 h post-blood meal (PBM) vitellogenic females. As shown in Fig. 2A, only CYP302A1 and CYP315A1 transcripts were detected in the ovaries of non-blood-fed (NBF) females. After the blood meal, a continuous expression of CYP302A1 was observed, whereas CYP306A1, CYP315A1, and CYP314A1 expression was detected only at 18 and 24 h PBM. CYP306A1 expression was restricted to the ovary whereas CYP302A1, CYP315A1, and CYP314A1 transcripts were also detected in other tissues. However, the concomitant expression of all steroidogenic enzymes was observed in the ovaries only at 18 and 24 h PBM, suggesting that only ovaries are able to produce 20E after a blood meal. In situ hybridization results for CYP302A1 were consistent with the RT-PCR analysis (see Fig. S2).
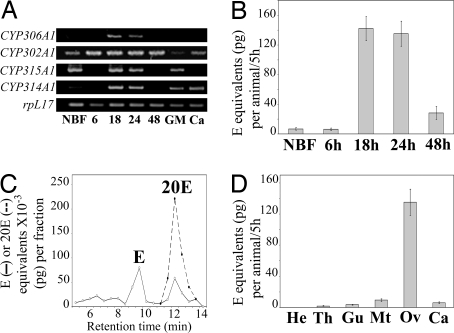
Fig. 2.
Ovaries produce 20E after blood meal in A. gambiae. (A) RT-PCR analysis of CYP306A1, CYP302A1, CYP315A1, and CYP314A1 expression pattern in ovaries throughout the first gonotrophic cycle (NBF, ovaries from non-blood-fed females; 6, 18, 24, and 48 h, ovaries at different times PBM) and in different body parts of 24 h PBM blood-fed females (GM, gut and Malpighian tubules; Ca, carcass). (B) In vitro ecdysteroid secretion by ovaries during the first gonotrophic cycle. (NBF, ovaries from non-blood-fed females; 6, 18, 24, and 48 h, ovaries from blood-fed females at different times PBM.) (C) HPLC-EIA analysis of ecdysteroids secreted by ovaries of blood-fed females (24 h PBM). Results are expressed as E (solid line) and 20E (dotted line) equivalents. (D) In vitro ecdysteroid secretion by different body parts of blood-fed females (24 h PBM). (He, head; Th, thorax; Gu and Mt, gut and Malpighian tubules; Ov, ovaries; Ca, carcass.)
We then investigated ovarian steroidogenic capacities of 5-day-old A. gambiae females during their first gonotrophic cycle. The ecdysteroid in vitro secretion of ovaries from NBF females and from females collected at 6, 18, 24, and 48 h PBM was measured by enzyme immunoassay (EIA). As shown in Fig. 2B, ovarian ecdysteroid secretion was hardly detectable before or shortly after (6 h PBM) ingestion of the blood meal. It reached a maximum at 18 and 24 h PBM, with a secretion of 142 and 135 pg E equivalents/ovary pair/5 h, respectively. At 48 h PBM, ecdysteroid secretion had declined to 28 pg E equivalents/ovary pair/5 h. HPLC-EIA analysis of incubation media revealed that 24 h PBM ovaries secrete both E and 20E (Fig. 2C). Taking into account the different affinities of the polyclonal L2 antibody for E and 20E, the ovaries secrete a 1:3 mixture of E and 20E. No ecdysteroids could be detected in 24 h PBM ovaries' extracts before or after incubation in vitro, indicating that ecdysteroids are readily secreted after synthesis and that no free E or 20E is stored inside the ovaries under our experimental conditions (data not shown). As CYP302A1, CYP315A1, and CYP314A1 transcripts were detected in other female tissues at 24 h PBM, we analyzed in vitro ecdysteroid secretion by those tissues (Fig. 2D). Ecdysteroid secretion by head, thorax, gut, Malpighian tubules, or carcass of 24 h PBM females was always below or at the detection limit of the EIA, in contrast to ovaries. Our results indicate that A. gambiae ovaries are the main female steroidogenic organs that produce and secrete 20E after blood feeding, a situation similar to what has been described for Aedes aegypti (11, 28, 29). In addition, transcript levels of the enzymes closely parallel the steroidogenic capacities of ovaries throughout the ovarian cycle, suggesting that steroidogenesis is regulated at the transcriptional level. Similar observations have been reported for the A. aegypti ovary as well as for the prothoracic glands of D. melanogaster and Bombyx mori during larval development (22, 28, 30).
Male Accessory Glands Produce and Store High Amounts of 20E.
In mosquitoes, the male reproductive tract (MRT) consists of a pair of testes connected by genital ducts to seminal vesicles and an ejaculatory pump. Adjacent to the seminal vesicles are two large accessory glands (i.e., MAGs), which unite at their bases and open into the anterior end of the ejaculatory pump (29). Expression of the four steroidogenic enzymes was examined by RT-PCR in testes, MAGs plus seminal vesicles and ejaculatory pump, digestive tract, and carcass from 6-day-old adults. As shown in Fig. 3A, the four transcripts were highly expressed in MAGs only. A minor expression of CYP314A1 was nevertheless detected in the testes. Further analysis by in situ hybridization revealed that expression of the four genes is mostly restricted to the anterior columnar epithelial cells of the MAGs (Fig. 3 B and C; data not shown). The epithelium of MAGs is indeed composed of two types of cells, which differ in their secretions: cells from the anterior part release lipidic secretions in the gland lumen by an apocrine process in contrast to cells from the posterior part, which release their proteic secretions by a holocrine process (8). Taken together, these results strongly suggested that A. gambiae MAGs, but neither testes nor seminal vesicles, have the ability to produce ecdysteroids.
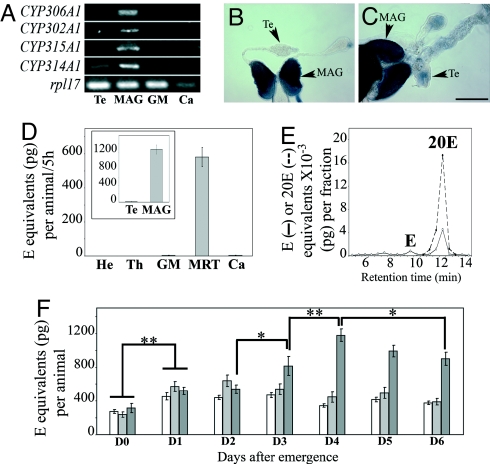
Fig. 3.
Male accessory glands produce 20E in A. gambiae. (A) RT-PCR analysis of CYP306A1, CYP302A1, CYP315A1, and CYP314A1 expression pattern in different body parts of 6-day-old males. (Te, testes; GM, gut and Malpighian tubules; Ca, carcass.) In this carcass sample, expression level of the control gene RpL17 was lower than in the other tissues examined; replicate experiments confirmed that expression of the steroidogenic genes was at the detection level limit. (B and C) In situ expression pattern of CYP306A1 (B) and CYP314A1 (C) in MRT from 6-day-old males (CYP302A1 and CYP315A1 not shown). (Te, testis; Sb, 250 μm.) (D) In vitro ecdysteroid secretion by different body parts of 6-day-old males. (He, head; Th, thorax; GM, gut and Malpighian tubules; Ca, carcass.) (Inset) In vitro ecdysteroid secretion by separated testes (Te) and MAG of 6-day-old males. (E) HPLC-EIA analysis of ecdysteroids secreted by MRT of 6-day-old males. Results are expressed as E (solid line) and 20E (dotted line) equivalents. (F) Ecdysteroid titers in MRT (white bars), ecdysteroid titers in whole males (light gray bars), and in vitro ecdysteroid secretion by MRT in 5 h of incubation (dark gray bars) from day 0 (D0) to day 6 (D6) PE (*, P < 0.05; **, P < 0.01).
To determine whether the MRT is actually producing ecdysteroids in the male malaria mosquito, we measured the ecdysteroid secretion in vitro by different body parts of 6-day-old males using EIA (Fig. 3D). MRT released a large amount of ecdysteroids, whereas no ecdysteroids were detected in the incubation media of the other male tissues. The amount of ecdysteroids secreted by MRTs varied from one mosquito generation to the other but was always higher than the amount secreted by steroidogenic ovaries. Further in vitro experiments showed that, within the MRT, only MAGs secrete substantial amounts of ecdysteroids, whereas testes produce at most tiny amounts (Fig. 3D Inset). Thus, only MAGs are steroidogenic in males at day 6 post-emergence (PE), confirming the RT-PCR results. HPLC-EIA analyses showed that MAGs produce and secrete mainly 20E, which is the vitellogenic steroid hormone (Fig. 3E). Additionally, although high amounts of ecdysteroids (927 ± 257 pg E equivalents/MRT/5 h) were found in the medium after incubation, similar amounts of ecdysteroids were detected in MRT extracts before or after incubation (445 ± 48 and 411 ± 48 pg E equivalents/MRT, respectively). This indicates that MAGs are capable of very high de novo synthesis of ecdysteroids during incubation. However, it was striking to find very high levels of free ecdysteroids in MAGs as E and 20E are diffusible molecules. Overall, these results raised the question of the biological significance of this high 20E production in males of A. gambiae, as the function of steroidogenesis has not been established in male insects.
To better appraise the temporal variations in ecdysteroid production, storage, and secretion during early adult life, we measured ecdysteroid levels in whole virgin males and in MRT of virgin males, as well as the in vitro steroidogenic capacities of MRT from day 0 to day 6 PE (Fig. 3F). Soon after emergence (i.e., D0), significant amounts of 20E were already stored in MRT of virgin males. These amounts of 20E stored in MRT increased during the first 2 days PE and then remained fairly constant throughout adult life. Surprisingly, a comparison of ecdysteroids amounts in MRT and in whole males revealed no significant differences despite the high secretion capacity of MRT observed in vitro. This means that, in vivo, nearly all 20E remains stored in the MRT and that, at most, a small percentage of the 20E is released into the hemolymph of the male. In vitro, the genital apparatus was already able to produce and secrete high amounts of ecdysteroids at emergence (i.e., D0). This in vitro ecdysteroid secretion capacity increased significantly from D0 to D1 and remained similar at D2. It then strongly increased at D3 to reach maximum values at D4 and slowly decreased until D6, returning back to D3 levels. Thus, our findings show that virgin males produce 20E and fill their MAGs with this hormone during the first few days of adult life, and that 20E amounts remain constant thereafter. Moreover, no or little 20E is secreted in the whole male compared with the level in MAGs. The high steroidogenic capacities of MAGs observed in vitro suggest that, in vivo, a negative feedback, the nature of which has yet to be identified, controls MAG ecdysteroid production. Interestingly, steroidogenic capacities of MAGs dramatically increase when males become sexually mature at D3 and can mate.
The 20E Stored in MAGs Is Delivered to Females During Mating.
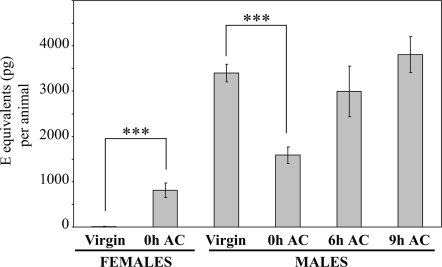
In mosquitoes, as in other insects, not only sperm, but also secretions produced and stored by MAGs are transferred to the female during mating, and can affect the physiology and behavior of the female, evoking, e.g., sexual refractoriness, modulation of host-seeking behavior, blood digestion, egg development, or oviposition (8, 9). Because MAG secretions in Anopheles are largely depleted during mating (ref. 31 and E.P., unpublished work), it was tempting to speculate that the high amount of 20E stored in MAGs would be transferred to the female during mating. To test this hypothesis, mating pairs of mosquitoes were caught one by one at D3 PE during copulation and partners were immediately placed on ice when copulation was achieved for subsequent quantification of 20E in each individual mosquito by EIA. The 20E was also measured in males maintained at 25 °C 6 h and 9 h after mating. As control, we measured 20E titers in whole virgin males or females that had been separated from emergence by a net to prevent copulation while permitting pheromonal interactions. Detection of spermatozoa in the spermathecae of captured mating females was used as the signature of successful copulation. Under our experimental design, 100% of captured mating females had a spermathecae full of spermatozoa (data not shown). Results presented in Fig. 4 show that, as expected, virgin females did not contain detectable amounts of 20E. In contrast, inseminated females contained significantly more 20E (Mann-Whitney test, P < 0.0001). Because females were killed few seconds after mating, it is very unlikely that de novo synthesis significantly contributed to these remarkable amounts of 20E detected in mated females. Moreover, this 20E increase in mated females matched exactly with the 20E decrease in the cognate mated males compared with virgin males of the same age and generation (P < 0.0001). Few hours after mating, males exhibited the same 20E levels as before mating. From these experiments it can be concluded that males of A. gambiae transferred high amounts of 20E to females during mating and that males are subsequently able to refill their glands with 20E within a few hours.
Fig. 4.
Males transfer 20E to females during mating. Ecdysteroid titers in virgin females (n = 15), in mated females just after copulation (0 h AC, n = 15), in virgin males (n = 15), in mated males just after copulation (0 h AC, n = 15), and in mated males 6 h (n = 9) and 9 h (n = 12) after copulation (6 h AC; 9 h AC). Ecdysteroids were extracted from each individual mosquito and quantified by EIA. Results are expressed as mean ± SEM in E equivalents (in pg) per animal. Results were subjected to statistical analysis using Mann-Whitney test (***, P < 0.0001).
Discussion
It was in a mosquito that Hagedorn and collaborators discovered, in 1975, secretion of steroids by insect ovaries (11). More than 30 years later, our results show that A. gambiae males produce large amounts of 20E, a major hormone previously described as the female sex steroid for mosquitoes and other insects. Furthermore, we provide evidence that the production of 20E in A. gambiae males is restricted to its reproductive accessory glands and that this steroid hormone is transferred to females during mating. In addition, the steroidogenic capacity of male accessory glands even exceeds the one of vitellogenic ovaries.
Since the pioneer work of Hagedorn and collaborators in A. aegypti (11), the production of 20E by adult females has been established in several insect species including various mosquito species (12, 13, 29, 32, 33, 34). In the studied mosquito species, 20E production is tightly linked to the gonotrophic cycle. A. gambiae female does not depart from this picture, as they also produce 20E after a blood meal, as reported here. Furthermore, we have demonstrated that the enzymes involved in steroidogenesis are functionally conserved between A. gambiae and other insect species (21–27), and that ecdysteroids are produced and secreted by ovaries in Anopheles female mosquitoes, as described for A. aegypti (11). By contrast, the A. gambiae male mosquitoes appear so far unique among insects by their high production and storage of the steroid hormone 20E. Indeed, in several dipteran species including Drosophila, no ecdysteroids have been evidenced in males (20, 35). In the few other insect species that were assessed for 20E production during male adulthood, steroid titers were much lower than in adult females and no prominent compound was identified (19). Whether the situation reported here for A. gambiae could be extended to other mosquito species has yet to be demonstrated. Indeed, although adult males of A. aegypti expressed some of the genes of the ecdysteroid biosynthetic pathway, they did not seem to express the genes encoding key enzymes for producing E and 20E (28), indicating that males of this species likely do not synthesize 20E. Although 20E is known in the female mosquito to stimulate vitellogenesis during a gonotrophic cycle, its endocrine function in adult male is elusive (as described later).
The high production of 20E by A. gambiae male mosquitoes is restricted to their MAGs. No 20E could be detected in testes or other body parts of the insect, including hemolymph, as similar amounts were found in extracts from whole mosquitoes and in isolated MAGs. This synthesis of steroids by male accessory glands but not testes is in sharp contrast with the situation observed in vertebrates. Our analyses reveal that 20E is produced from the beginning of the adult male life, reaching its highest in vivo levels from day 1 to day 3 PE. This period corresponds to the time required for male mosquitoes to acquire their sexual maturity before a first successful mating can take place (8). Interestingly, in vitro synthesis capacity of MAGs increased from day 3 PE and onward, which corresponds to the transition from acquired sexual maturity to sexual activity (36). The 20E production and storage in MAGs therefore appears to be an integrant part of the sexual maturation process. Furthermore, the constant amount of 20E detected in sexually matured animals or dissected MAGs compared with the in vitro synthesis capacity of this organ suggests that, in vivo, production of 20E is under the control of a negative regulatory mechanism. The nature of this retro-control and whether it is specific to steroidogenesis or common for all MAG secretions is yet unknown.
The most relevant role for the high 20E production in A. gambiae males is provided by our data showing that males transfer high quantities of this steroid hormone to females during mating. Furthermore, in accordance with the high in vitro steroidogenic capacities of MAGs, males are able to re-synthesize 20E soon after mating for a subsequent mating event, as described for other MAG products (8, 31). Although it is common that males invest more in mating than in parenting, contrary to females, males of many species have developed paternal investment, which can be costly for them, either to ensure their paternity or to benefit the offspring (37, 38). Indeed, in many insects, transfer of male semen during mating induces striking changes in the behavior and physiology of females. Mated females can become temporarily refractory to subsequent matings and can increase egg development or oviposition (8, 9, 39). While these post-mating changes triggered in females are well conserved in insects, genes and physiological processes mediating these effects are species-specific as a result of the high divergence of insect reproductive systems (40). In D. melanogaster, a MAG effect mediated by various accessory gland proteins (Acps), including a sperm effect mediated by the sex peptide (one of the Acps), has been elegantly demonstrated using genetics (41, 42). In mosquitoes, MAG secretions transferred to females during copulation have also been implicated in the female post-mating effects. In A. aegypti, a MAG effect is mediated by the matrone peptide, which may stimulate vitellogenesis (9). Many Acps, which have been recently identified in Aedes males and mated females, are also suspected to influence female after mating (43). Additionally, JH, which is involved in ovarian follicle development to the pre-vitellogenic resting stage in female mosquitoes, is synthesized and stored in Aedes MAGs and possibly transferred to the female during mating (44). In Anopheles, mating-induced changes in female mediated by MAG secretions have also been demonstrated (45, 46), but the mediators remained uncharacterized. A recent genome-wide blast analysis has identified Anopheles Acps specifically expressed in MAGs and possibly involved in the modulation of post-mating effects in Anopheles females (47). Thus, in association with Acps, the steroid hormone 20E offered by the male may play a crucial role in eliciting post-mating physiological changes in A. gambiae females.
Among the post-mating physiological changes, it is tempting to speculate that 20E transferred by males may stimulate vitellogenesis and/or ovarian development in mated females. In female mosquitoes, whether 20E is produced by the ovaries or injected into the hemolymph, 20E triggers production of vitellogenin proteins by the fat body cells. This has been well documented in A. aegypti (11, 12, 13, 29, 48) and extended to some Anopheles species (32, 33). According to the tight link between blood-feeding and vitellogenin expression in A. gambiae (49), and the 20E production by vitellogenic ovaries shown in the present study, it is very likely that this regulation also applies to A. gambiae, although this has not been formally demonstrated. Then, 20E transferred by males may contribute to trigger vitellogenesis in females. However, because A. gambiae females mate generally only once in their life (8) and 20E is both diffusible and short-lived, 20E transferred by males during mating might rather contribute to ovarian maturation in nulliparous females. Indeed, injection of 20E in nulliparous non-blood-fed females has been reported to induce the separation of the secondary follicles from the germarium in both A. aegypti and Anopheles stephensi (33, 50). In our A. gambiae samples, we observed that the secondary follicles have separated or were close to separation from the germarium in most virgin and mated non-blood-fed female ovaries, indicating that 20E provided by males during mating does not trigger the separation of the secondary follicle (data not shown). Clearly, more experiments and tools are needed to determine the role of the 20E transfer from males to females during mating and whether and how 20E is released from the female genital tract.
In conclusion, A. gambiae adult males appear so far unique for their high production and storage of 20E, the vitellogenic steroid hormone, in their MAGs. Moreover, male 20E is transferred to females during mating; to our knowledge, this is the first description of a sexual transfer of steroid. This suggests strongly that male 20E acts rather as an allohormone (defined in ref. 51), influencing the female reproduction and indirectly increasing the male reproductive success, than as a true male steroid hormone, even if we cannot presently exclude endogenous effects of ecdysteroids at low concentration. In any case, our results emphasize the rapid evolution of reproductive systems in insects and show the importance of studying hormonal processes governing the reproduction in disease vectors. Indeed, these results may further provide new bases for the control of the malaria vector through decreasing female fecundity by manipulating male reproductive genes.
Materials and Methods
Mosquito Rearing and Manipulation.
Larvae of A. gambiae (Yaoundé strain) were reared at 25 °C in deionized and distilled water on TetraMin Baby-E fish food from the day of hatching to the fourth larval instar. A. gambiae male and female adults were maintained at 25 °C, under conditions of 68% relative humidity and a 12/12 h light/dark cycle, on 10% wt/vol sucrose solution for the first 5 days PE. For mass rearing, female mosquitoes (first gonotrophic cycle) were allowed to feed on the blood of an anesthetized rabbit for 30 min. Tissues were carefully dissected from NBF females or from females at different times after the blood meal (partially fed females were discarded). Virgin males were dissected from day 0 to day 6 PE. Mating experiments were conducted as follows: mating pairs were caught in the rearing cages one by one during copulation using an aspirator, and individuals were separated as soon as copulation was achieved. Each male and cognate female were immediately placed separately on ice. Detection of spermatozoa in the spermathecae of captured females was used as the signature of successful copulation. Independent cohorts of mating pairs were used to quantify ecdysteroids by EIA. Ecdysteroids were extracted from each individual whole male and female transferred into methanol.
A. gambiae Steroidogenic Enzyme Gene Cloning.
Total RNA was isolated with TRIzol reagent (Invitrogen) from whole late fourth instar larvae and reverse transcribed with M-MLV reverse transcriptase (Promega). The A. gambiae genome is sequenced and genomic data are available online (http://www.ensembl.org/). In addition, various insects' P450s genomic data may also be found online (http://p450.antibes.inra.fr/). Full-length cDNA sequences of AgCYP306A1, AgCYP302A1, AgCYP315A1, and AgCYP314A1 were amplified from total cDNAs by PCR with specific primers (Table S1). cDNAs were gel-purified and cloned into pGEM-T Easy vector using a TA cloning kit (Promega), and insert sequences were verified (Genome Express). For transfection experiments, AgCYP302A1, AgCYP315A1, and AgCYP314A1 ORFs were further sub-cloned in pCaSpeR-actin-EcoRI (52) or in pIB/V5-His (TA cloning; Invitrogen).
RT-PCR.
Tissues were carefully dissected in ice-cold, RNase-free PBS solution (100 mM, pH 7.4), containing 0.1% Tween (i.e., phosphate buffer Tween [PBT]). Total RNA was then extracted with SV Total RNA Isolation System (Promega) and quantified by spectrophotometry at 260 nm. cDNAs were generated using M-MLV reverse transcriptase from 350 ng (female tissues) and from ≈15 ng (male tissues) total RNA. The number of PCR cycles was adjusted depending on the concerned gene and experimental series (female tissues, CYP306A1, 45 cycles; CYP302A1, 35 cycles; CYP315A1, 36 cycles; CYP314A1, 40 cycles; RpL17, 24 cycles; male tissues, CYP306A1, 48 cycles; CYP302A1, 33 cycles; CYP315A1, 38 cycles; CYP314A1, 37 cycles; RpL17, 33 cycles). RpL17, coding for the ribosomal protein L17, is a domestic gene used as internal control. Primers used for RT-PCR experiments are given in Table S1.
Whole-Mount RNA in Situ Hybridization.
Ovaries and MRTs were carefully dissected in PBT and fixed with 4% paraformaldehyde. In situ hybridization was carried out according to the method described by Parvy et al. (30). Sense and antisense ribo-probes were synthesized from AgCYP306A1, AgCYP302A1, AgCYP315A1, and AgCYP314A1 full-length cDNA cloned into pGEMT-easy.
In Vitro Organ Culture.
After dissection in PBT, tissues were rinsed separately in Schneider medium (Gibco-BRL) and preincubated for 30 min in 50 μl fresh medium under gentle horizontal agitation at 25 °C. Tissues were then transferred into 50 μl new medium and incubated for a further 5 h at 25 °C. After incubation, culture medium and tissues were collected separately and stored at −20 °C until ecdysteroid quantification.
Ecdysteroid Quantification.
Ecdysteroids were quantified by EIA, with 20-hydroxyecdysone-2-succinate coupled to peroxidase as a tracer (dilution 1:80,000) and the L2 antiserum (gift from M. De Reggi [Marseille, France]; dilution, 1:40,000). This antibody recognizes both E and 20E, with a 3.8-fold higher affinity for E than for 20E, as calculated from the comparison of reference standard curves. Calibration curves were generated with E (3.6–500 pg/tube) and 20E (16–2,000 pg/tube) diluted in EIA buffer or Schneider medium, and titers were expressed as either E or 20E equivalents. In these conditions, detection limit is 9 pg E equivalents. Ecdysteroids secreted by tissues were measured directly on incubation media. Calibration curves were generated with E diluted in Schneider medium and the in vitro production was expressed in E equivalents. Total ecdysteroids from ovaries, male gonads, or whole animals were extracted with methanol and re-dissolved in EIA buffer. Ecdysteroids were measured by using an E calibration curve diluted in EIA buffer. All measurements were performed in duplicate and the results are expressed as mean values ± SEM. of several (n = 12) independent samples and have been repeated on at least two independent cohorts of mosquitoes. Samples at or above the highest value of the calibration curve were diluted and quantified again. The intra- and inter-assay variation coefficients were 4% and 6.5%, respectively. Data were subjected to statistical analysis using Mann-Whitney and ANOVA Kruskal-Wallis tests.
Chromatographic Analyses.
To identify the ecdysteroids released by the gonads, representative in vitro incubation media were pooled; ecdysteroids were adsorbed on to C18 Sep-Pak cartridges (Waters) and eluted in methanol. Samples were then subjected to normal-phase HPLC (Zorbaxa SIL; 250 × 4.6 mm, 5 μm) using dichloromethane-isopropanol-water (100:40:2.5, vol/vol/v; isocratic, 1 ml/min). Fractions were collected every 0.5 min during 20 min, evaporated to dryness, and re-dissolved in EIA buffer. Ecdysteroids were then measured in each fraction by EIA. The chromatographic mobility of immunoreactive material was compared with that of reference compounds.
Supplementary Material
Acknowledgments.
We thank Emilie Guittard (L.B.S.) for technical assistance and Marie-Thérèse Lecoq (CEPIA) for A. gambiae rearing and for her observations of mosquito behavior. We also thank Christian Roussilhon (Parasitologie Biomedicale, Institut Pasteur, Paris, France) for his help in biostatistics. We are very grateful to Prof. René Lafont and Dr. Jean-Philippe Parvy for helpful discussions and critical comments on the manuscript. We address many thanks to Dr. Laurence Dinan for improvement of the paper's written style and Dr. Kenneth Vernick for critical reading of the manuscript. This work was supported by the Université Pierre et Marie Curie and the Ministère de la Recherche Scientifique (E.P., A.M., and C.D.-V.), by the Fondation pour la Recherche Médicale and a Fondation des Treilles award (to E.P.), and by the Institut Pasteur and the Fonds Dédié Sanofi-Aventis/Ministère de la Recherche “Combattre les Maladies Parasitaires” (to J.-C.J. and C.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. EF546762–EF546765).
This article contains supporting information online at www.pnas.org/cgi/content/full/0809264105/DCSupplemental.
References
- 1.Roll Back Malaria, World Health Organization, UNICEF. World Malaria Report 2005. Geneva: WHO; 2005. [Google Scholar]
- 2.Miller LH, Greenwood B. Malaria–a shadow over Africa. Science. 2002;298:121–122. doi: 10.1126/science.1078048. [DOI] [PubMed] [Google Scholar]
- 3.Coleman PG, Alphey L. Genetic control of vector populations: an imminent prospect. Trop Med Int Health. 2004;9:433–437. doi: 10.1111/j.1365-3156.2004.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.Alphey L, et al. Malaria control with genetically manipulated insect vectors. Science. 2002;298:119–121. doi: 10.1126/science.1078278. [DOI] [PubMed] [Google Scholar]
- 5.Catteruccia F. Malaria vector control in the third millennium: progress and perspectives of molecular approaches. Pest Manag Sci. 2007;63:634–40. doi: 10.1002/ps.1324. [DOI] [PubMed] [Google Scholar]
- 6.Christophides GK. Transgenic mosquitoes and malaria transmission. Cell Microbiol. 2005;7:325–333. doi: 10.1111/j.1462-5822.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- 7.Benedict MQ, Robinson AS. The first release of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 2003;19:349–355. doi: 10.1016/s1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 8.Clements AN. The biology of mosquitoes: sensory reception and behaviour. 2nd Ed. Wallingford, UK: CABI; 1999. pp. 333–402. [Google Scholar]
- 9.Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu Rev Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 10.Holt RA, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- 11.Hagedorn HH, et al. The ovary as a source of α-ecdysone in an adult mosquito. Proc Natl Acad Sci USA. 1975;72:3255–3259. doi: 10.1073/pnas.72.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raikhel AS, Brown MR, Belles X. Hormonal control of reproductive processes. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 3. Oxford, UK: Elsevier; 2005. pp. 433–491. [Google Scholar]
- 13.Swevers L, Raikhel AS, Sappington TW, Shirk P, Iatrou K. Vitellogenesis and post-vitellogenic maturation of the insect ovarian follicle. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 3. Oxford, UK: Elsevier; 2005. pp. 87–155. [Google Scholar]
- 14.Loeb MJ, Woods CW, Brandt EP, Borkovec AB. Larval testes of the tobacco budworm: a new source of insect ecdysteroids. Science. 1982;218:896–897. doi: 10.1126/science.218.4575.896. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis TD, Earley FGP, Rees HH. Ecdysteroid biosynthesis in larval testes of Spodoptera littoralis. Insect Biochem Mol Biol. 1993;24:531–537. [Google Scholar]
- 16.Koolman J, Scheller K, Bodenstein D. Ecdysteroids in the adult male blowfly Calliphora vicina. Experientia. 1979;35:134–135. [Google Scholar]
- 17.Gillot C, Ismail PM. In vitro synthesis of ecdysteroid by the male accessory reproductive glands, testis and abdominal integument of the adult migratory grasshopper, Melanoplus sanguinipes. Invert Reprod Dev. 1995;27:65–71. [Google Scholar]
- 18.De Loof A, Huybrechts R. “Insects do not have sex hormones”: a myth? Gen Comp Endocrinol. 1998;111:245–260. doi: 10.1006/gcen.1998.7101. [DOI] [PubMed] [Google Scholar]
- 19.De Loof A. Ecdysteroids: the overlooked sex steroids of insects? Males: the black box. Insect Sci. 2006;13:325–338. [Google Scholar]
- 20.Lafont R, Dauphin-Villemant C, Warren JT, Rees H. Ecdysteroid chemistry and biochemistry. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 3. Oxford, UK: Elsevier; 2005. pp. 125–195. [Google Scholar]
- 21.Niwa R, et al. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J Biol Chem. 2004;279:35942–35949. doi: 10.1074/jbc.M404514200. [DOI] [PubMed] [Google Scholar]
- 22.Warren JT, et al. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol. 2004;34:991–1010. doi: 10.1016/j.ibmb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Chavez VM, et al. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development. 2000;127:4115–4126. doi: 10.1242/dev.127.19.4115. [DOI] [PubMed] [Google Scholar]
- 24.Warren JT, et al. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci USA. 2002;99:11043–11048. doi: 10.1073/pnas.162375799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petryk A, et al. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci USA. 2003;100:13773–13778. doi: 10.1073/pnas.2336088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niwa R, et al. The ecdysteroidogenic P450 Cyp302a1/disembodied from the silkworm, Bombyx mori, is transcriptionally regulated by prothoracicotropic hormone. Insect Mol Biol. 2005;14:563–571. doi: 10.1111/j.1365-2583.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 27.Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. Identification, characterization and developmental expression of Halloween genes encoding P450 enzymes mediating ecdysone biosynthesis in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2006;36:188–199. doi: 10.1016/j.ibmb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Sieglaff DH, Duncan KA, Brown MR. Expression of genes encoding proteins involved in ecdysteroidogenesis in the female mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2005;35:471–490. doi: 10.1016/j.ibmb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Clements AN. The biology of mosquitoes: development, nutrition and reproduction. New York: Chapman and Hall; 1992. [Google Scholar]
- 30.Parvy JP, et al. A role for βFTZ-F1 in regulating ecdysteroid titers during post-embryonic development in Drosophila melanogaster. Dev Biol. 2005;282:84–94. doi: 10.1016/j.ydbio.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 31.Mahmood F, Reisen WK. Anopheles culicifacies: effects of age on the male reproductive system and mating ability of virgin adult mosquitoes. Med Vet Entomol. 1994;8:31–37. doi: 10.1111/j.1365-2915.1994.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 32.Lu YH, Hagedorn HH. Egg development in the mosquito Anopheles albimanus. Int J Inv Reprod Dev. 1986;9:79–94. [Google Scholar]
- 33.Redfern CPF. 20-hydroxyecdysone and ovarian development in Anopheles stephensi. Insect Physiol. 1982;28:97–109. [Google Scholar]
- 34.Garen A, Kauvar L, Lepesant JA. Roles of ecdysone in Drosophila development. Proc Natl Acad Sci USA. 1977;74:5099–5103. doi: 10.1073/pnas.74.11.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bownes M, Dübendorfer A, Smith T. Ecdysteroids in adult males and females of Drosophila melanogaster. J Insect Physiol. 1984;30:823–830. [Google Scholar]
- 36.Verhoek BA, Takken W. Age effects on the insemination rate of Anopheles gambiae s. l. in the laboratory. Entomol Exp Appl. 1994;72:167–172. [Google Scholar]
- 37.Geary DC. Evolution of paternal investment. In: Buss DM, editor. The evolutionary psychology handbook. Hoboken, NJ: John Wiley; 2005. pp. 483–505. [Google Scholar]
- 38.Kvarnemo C. Evolution and maintenance of male care: is increased paternity a neglected benefit of care? Behav Ecol. 2006;17:144–148. [Google Scholar]
- 39.Klowden MJ, Russell RC. Mating affects egg maturation in Anopheles gambiae Giles (Diptera: Culicidae) J Vector Ecol. 2004;29:135–139. [PubMed] [Google Scholar]
- 40.Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nat Rev Genet. 2002;3:137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- 41.Chapman T, et al. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci USA. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sirot LK, et al. Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect Biochem Mol Biol. 2008;38:176–189. doi: 10.1016/j.ibmb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borovsky D, Carlson DA, Hancock RG, Rembold H, Van Handel E. De novo biosynthesis of juvenile hormone III and I by the accessory glands of the male mosquito. Insect Biochem Mol Biol. 1993;24:437–444. doi: 10.1016/0965-1748(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 45.Bryan JH. Results of consecutive matings of female Anopheles gambiae species B with fertile and sterile males. Nature. 1968;218:489. doi: 10.1038/218489a0. [DOI] [PubMed] [Google Scholar]
- 46.Bryan JH. Further studies on consecutive matings in the Anopheles gambiae complex. Nature. 1972;239:519–520. doi: 10.1038/239519a0. [DOI] [PubMed] [Google Scholar]
- 47.Dottorini T, et al. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc Natl Acad Sci USA. 2007;104:16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klowden MJ. Physiological systems in insects. 2nd ed. Amsterdam: Elsevier; 2007. p. 688. [Google Scholar]
- 49.Nirmala X, Marinotti O, James AA. The accumulation of specific mRNAs following multiple blood meals in Anopheles gambiae. Insect Mol Biol. 2005;14:95–103. doi: 10.1111/j.1365-2583.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 50.Beckemeyer EF, Lea AO. Induction of follicle separation in the mosquito by physiological amounts of ecdysterone. Science. 1980;209:819–820. doi: 10.1126/science.209.4458.819. [DOI] [PubMed] [Google Scholar]
- 51.Koene JM, Maat A. “Allohormones”: a class of bioactive substances favoured by sexual selection. J Comp Physiol A. 2001;187:323–326. doi: 10.1007/s003590100214. [DOI] [PubMed] [Google Scholar]
- 52.Thummel CS, Boulet AM, Lipshitz HD. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988;74:445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.