Abstract
Although the importance of cytotoxic T lymphocytes and neutralizing antibodies for antiviral defense is well known, the antiviral mechanism of Th1 remains unclear. We show that Th1 cells mediate noncytolytic antiviral protection independent of direct lysis through local secretion of IFN-γ after herpes simplex virus (HSV) 2 infection. IFN-γ acted on stromal cells, but not on hematopoietic cells, to prevent further viral replication and spread throughout the vaginal mucosa. Importantly, unlike other known Th1 defense mechanisms, this effector function did not require recognition of virally infected cells via MHC class II. Instead, recall Th1 response was elicited by MHC class II+ antigen-presenting cells at the site of infection. Dendritic cells (DCs) were not required and only partially sufficient to induce a recall response from memory Th1 cells. Importantly, DCs and B cells together contributed to restimulating memory CD4 T cells to secrete IFN-γ. In the absence of both DCs and B cells, immunized mice rapidly succumbed to HSV-2 infection and death. Thus, these results revealed a distinct mechanism by which memory Th1 cells mediate noncytolytic IFN-γ–dependent antiviral protection after recognition of processed viral antigens by local DCs and B cells.
One of the hallmarks of the adaptive immune system is its ability to provide long-term protection against infection by otherwise lethal pathogens. Great efforts have been placed into understanding the effector mechanisms that are capable of preventing diseases caused by infection. For viral infections, a vast majority of studies have focused on the role of CTL and neutralizing antibody (Ab) responses. A critical importance of these antiviral effectors in eliminating viral pathogens has been manifested by the large number of evasion strategies used by viruses to subvert detection by CTLs and Abs. In fact, some viruses are so efficient at preventing detection by CTLs and Abs that these effectors are rendered incapable of providing protection in an immunized host (1), which is exemplified by infection with HIV-1 and γ-herpesvirus (2, 3). Alternative means of providing antiviral protection are required to combat infection by such viruses.
HSV-2, one of the most common sexually transmitted infections, causes primary infection in the genital mucosal epithelial layer and establishes latency in the sacral ganglia. In the mouse model of genital herpes, priming of the host with an attenuated thymidine kinase (TK) mutant HSV-2 via the intravaginal (ivag) route provides lifelong protection against challenge with virulent WT HSV-2. Such protection is mediated in a CD4 T cell–dependent manner (4, 5). In contrast, mice deficient in immunoglobulin or CD8 T cells are protected from virulent HSV-2 challenge after ivag immunization with TK−HSV-2 virus (4–7), suggesting that the protection requires CD4 T cells but not CTL or Ab responses. However, the precise mechanism by which the memory Th1 cells provide immune protection in the vaginal mucosa is unknown.
The importance of Th1 effector cells in defense against intracellular bacterial and protozoan pathogens has been well characterized (8, 9). This process primarily involves the activation of infected phagocytes through IFN-γ, resulting in enhanced phagocytosis and intracellular degradation of bacterial and protozoan pathogens. In contrast, the mechanisms by which Th1 memory cells provide protection against viruses remain much less clear (10, 11). There are at least three distinct mechanisms that can account for the ability of Th1 cells to mediate antiviral responses. The first is an indirect mechanism where Th1 cells are required for providing help to sustain effector CTL and B cells but do not themselves play a direct role in clearance of virus in vivo. Examples of this type of Th1 function has been seen in West Nile virus (12) and influenza virus infections (13). The second is the direct lysis of virally infected cells by Th1 killer cells. A recent study revealed the importance of antiviral Th1 cells in directly recognizing and killing influenza virus–infected cells through perforin-dependent pathways (14). In this study, it was shown that IFN-γ secretion by CD4 T cells was not required for their antiviral effector function. Direct recognition and lysis of infected B cells by CD4 T cells also plays an important role in control of Epstein Barr virus infection (15). A third mechanism involves antiviral function mediated by secreted factors. CD4 T cells secrete cytokines such as IFN-γ and TNF, which are known to control viral replication. Such a mechanism was shown to mediate viral clearance after the transfer of in vitro–derived Th1 cell against vesicular stomatitis virus (16) and in hepatitis B virus transgenic (Tg) mice (10). In the case of genital herpes infection, neutralization of IFN-γ (5, 17, 18) or genetic deficiencies in IFN-γ (4) render mice incapable of suppressing viral replication. However, the precise mechanism by which Th1 cells are elicited to secrete IFN-γ during the recall response is unknown.
A key question in this regard is whether Th1 cells are stimulated to secrete antiviral cytokines by direct recognition of virally infected cells through viral antigenic peptides presented on MHC class II or by an indirect mechanism through recognition of local APCs that have taken up viral antigens from infected cells. This issue is particularly relevant for infection by viruses, including HSV-2, that specifically replicate within non-APCs, namely, the mucosal epithelial cells (19). Most viruses target cells that do not normally express MHC class II. Previous studies have shown that in HSV-2–primed mice, up-regulation of MHC class II on vaginal epithelial cells occurs after secondary infection in an IFN-γ–dependent manner (7, 20), raising the possibility that nonprofessional APCs could present viral peptides to CD4 T cells directly and become the target of lysis by CD4 killer cells. Because one of the hallmarks of IFN-γ is its ability to induce MHC class II molecules in professional and nonprofessional APCs, it has been assumed that this enables infected cells to directly present viral antigens on MHC class II for recognition by Th1 cells. In fact, HSV infection leads to inhibition of MHC class II processing pathways, suggesting that direct recognition of infected cells by CD4 T cells is detrimental to viral spread in the host (21).
In this study, we examine the mechanism by which memory Th1 cells provide protection against genital HSV-2 infection in mice previously immunized with an attenuated TK−HSV-2. Specifically, we investigate the importance of the localization of memory Th1 cells, and the effector molecules and cell types involved in mediating Th1 antiviral protection in the mucosa in response to HSV-2 challenge.
RESULTS
Memory Th1 cells, but not CD8 T cells, localize in the vaginal mucosa and are required for virus clearance after HSV-2 secondary challenge
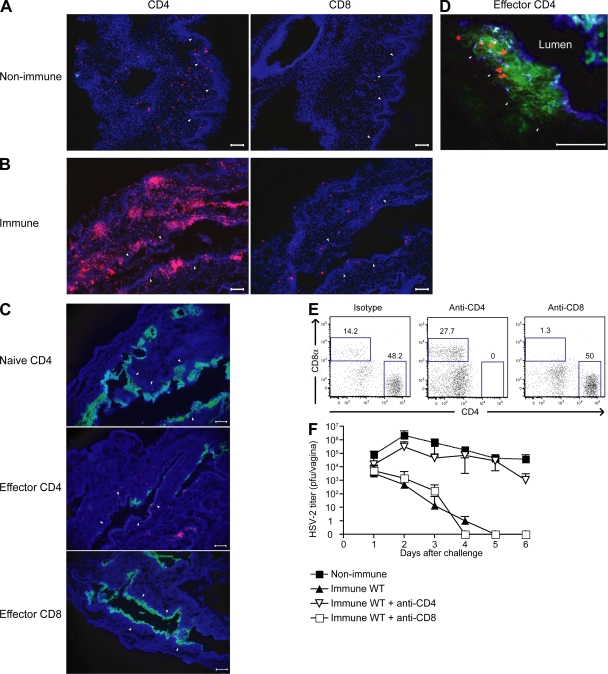
Ivag infection of naive mice with the neurovirulent WT HSV-2 (186 strain) virus leads to rapid replication in the vaginal epithelium followed by paralysis and death within 7–8 d (4, 7). However, mice previously immunized with an attenuated HSV-2 (TK− strain) are protected from secondary challenge with the WT 186 strain. To begin to elucidate the mechanism by which Th1 memory cells provide protection at the mucosal tissue, we examined the localization of CD4 T cells in the vaginae of naive and immune mice. In contrast to the scattered distribution of CD4 T cells in the vagina of naive mice (Fig. 1 A), organized clusters of CD4 T cells were evident in the vaginal submucosa of mice at 3 wk after immunization with TK−HSV-2 (Fig. 1 B). These CD4 T cells had an activated phenotype, as they expressed CD44 and CD11a (unpublished data). To examine the migratory ability of these T cell subsets to the vaginal mucosa, bulk effector CD4 or CD8 T cells purified from the draining LNs of TK−HSV-2–primed mice were adoptively transferred into congenic hosts. The localization of these cells was tracked by staining for the CD45.1 congenic marker of the donor T cells in situ. Upon infection of the host animals with HSV-2 ivag, only the effector CD4 and not naive CD4 or effector CD8, T cells were found to localize to the vagina (Fig. 1 C). Further analyses revealed that these CD4 T cells migrated above the basement membrane into the infected epithelial layer (Fig. 1 D). Subsequently, only the mice that received effector CD4 and not CD8 T cells were able to control viral replication and spread (Fig. 1 C). Next, the specificity of the migration of memory Th1 cells was examined (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20082039/DC1). Donor mice (CD45.1+) were infected ivag with HSV-2 or influenza virus, and the migration pattern of bulk effector LN CD4 T cells was followed upon adoptive transfer into congenic hosts that were infected with either HSV-2 or influenza virus. These analyses revealed that effector CD4 T cells migrated into the vaginal tissue only in response to homologous and not heterologous virus infection (Fig. S1). Thus, memory CD4 T cell migration is pathogen specific, and inflammatory condition caused by virus infection alone is not sufficient to recruit nonspecific CD4 T cells into the vaginal mucosa.
Figure 1.
Memory CD4 T cells localize in the vagina of immune mice and provide protection against secondary HSV-2 challenge. (A and B) The localization of CD4 and CD8 T cells in the vagina of nonimmunized (A) or TK−HSV-2–immunized mice at 3 wk after immunization (B). (C and D) Congenic (CD45.1+) effector CD4, CD8, or naive CD4 T cells were adoptively transferred into naive recipient (CD45.2+) mice. At 3 d after ivag infection with HSV-2, viral antigen (anti–HSV-2, green; C and D), transferred T cells (anti-CD45.1, red; C and D), and MHC class II (blue; D) were visualized. Images were captured using a 10 (A–C) or 40× (D) objective lens. Arrowheads indicate the basement membrane. Bars, 100 μm. (E and F) CD4 or CD8 T cells were depleted from TK−HSV-2–primed mice and challenged ivag with WT HSV-2. (E) The dot plot represents CD4+ and CD8+ cells in vagina 3 d after challenge. (F) Virus titers in the vaginal fluids (nonimmune, n = 4; immune/control Ab, n = 6; immune/anti-CD4, n = 4; and immune/anti-CD8, n = 4) were measured at the indicated days after HSV-2 secondary challenge. Error bars represent the mean ± SD of the number of mice per group. These data are representative of three similar experiments.
To further dissect the importance of memory CD4 T cells in providing protection against the secondary HSV-2 challenge, either CD4+ or CD8+ cells were depleted by Ab injection in HSV-2–immune animals before secondary challenge. After Ab injection, CD4+ or CD8+ T cells were completely depleted from the vagina (Fig. 1 E) and elsewhere (not depicted). After challenge with WT HSV-2, virus titers in vaginal wash of the mice treated with anti-CD4 Ab were significantly higher than isotype control IgG treated group (Fig. 1 F). In fact, the viral titers in the CD4-depleted immune mice were comparable to those in the naive animals infected with the WT HSV-2 for the first time, indicating that the protection in immune hosts was primarily mediated by the CD4 T cells. The anti-CD4 Ab treatment specifically depleted CD4 T cells but not CD4lo MHC II+ APCs in the vagina (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20082039/DC1). This is likely because of higher expression of CD4 molecule by the T cells compared with APCs (Fig. S2). In contrast, immune mice depleted of CD8 T cells were protected from secondary viral challenge in a manner comparable to that of the intact immunized animals. Although somewhat at odds with the study by Parr and Parr (7), these data are consistent with other previously published studies (4–6, 18), which demonstrated that memory Th1 cells, but not CD8 T cells, play a predominant role in virus clearance at the mucosal surface after genital HSV-2 challenge. This observation correlated with the formation of clusters of CD4 T cells, but not CD8 T cells, in the vaginal submucosa of the vaginae of immunized host and with the selective migration of adoptively transferred Th1 cells, but not CTLs, to the site of HSV-2 infection.
Direct lytic pathways involving perforin or Fas–FasL are not required for protection mediated by memory CD4 T cells after genital HSV-2 challenge
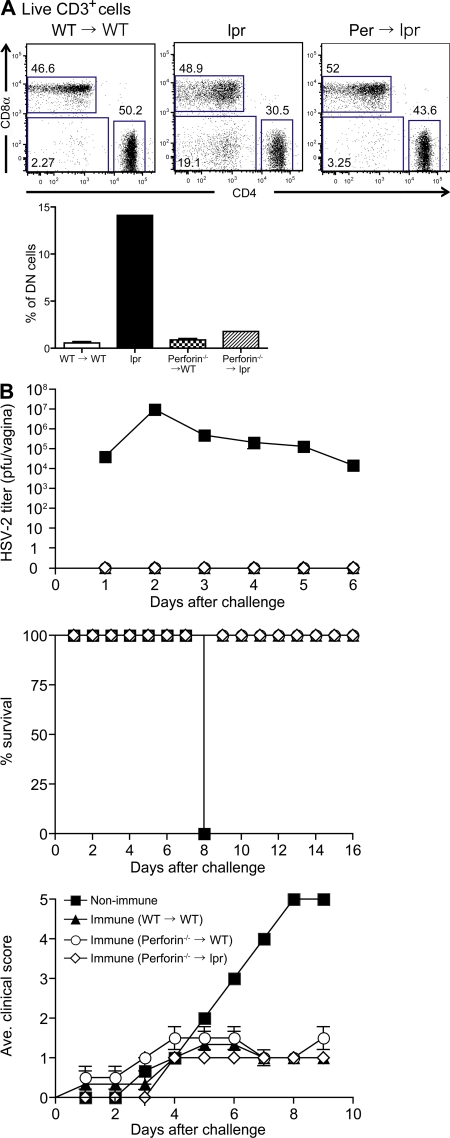
Clearance of virus infection by memory Th1 cells can be mediated either by direct lysis of infected cells or through a noncytolytic pathway. Perforin-mediated cytotoxic mechanisms have been seen in human CD4 T cell lines against HSV-infected cells (22), whereas both perforin- and Fas-dependent cell lysis are used by anti–HSV-2 CD8 T cells (23). To determine the collective requirement of the two major mechanisms to directly lyse virally infected cells, namely perforin and FasL, we generated BM chimeric mice in which lethally irradiated B6.MRL/lpr (Fas mutant) mice were reconstituted with BM cells isolated from Perforin−/−mice (Perforin−/−→lpr). A potential complication of the use of the lpr mice is the abnormal development of the CD4−CD8−B220+double negative (DN) T cells leading to lymphadenopathy (24). 14 wk after BM transplantation, the emergence of the DN T cells in draining LNs was analyzed by flow cytometry. Unlike the lpr mice (Fig. 2 A), the percentages of DN T cells in Perforin−/−→lpr chimera were comparable to the WT→WT and Perforin−/−→WT chimeric mice. Perforin−/−→lpr mice appeared to develop a normal T cell compartment devoid of autoreactive cells, circumventing problems associated with cell transfer into nonlethally irradiated lpr mice (25). Consistent with this, we observed no splenomegaly or lymphadenopathy in the Perforin−/−→lpr chimera (unpublished data). Thus, Perforin−/−→lpr mice allowed us to examine the collective importance of perforin- and Fas-FasL–mediated lysis of infected cells by Th1 cells in the absence of autoimmunity. TK−HSV-2–immunized Perforin−/−→ lpr mice were found to be completely resistant to secondary challenge with WT HSV-2 and were protected from viral disease in a manner comparable to that of the WT→WT and Perforin−/−→WT chimeric mice (Fig. 2 B). These data revealed that neither perforin nor Fas–FasL pathways were required for the Th1-mediated protection against HSV-2 challenge.
Figure 2.
Direct cytolysis via perforin or Fas–FasL is not required for Th1-mediated protection. C57BL/6 (WT) →WT (n = 3), Perforin−/−→WT (n = 4), and Perforin−/−→lpr chimera (n = 5) were immunized with TK−HSV-2 ivag and, 3 wk later, challenged with lethal WT HSV-2 virus. As a control, nonimmunized C57BL/6 mice were challenged with the virus. (A) FACS analyses of CD4 and CD8 expression in live CD3+ cells in the peripheral blood of WT →WT chimera, Perforin−/−→lpr chimera, and lpr mice. The percentage of the CD3+CD4−CD8−B220+ cells (DN cells) found in these mice is shown in the histogram. (B) Survival, genital mean pathology scores, and viral titers in vaginal wash after secondary challenge are depicted. Error bars represent the mean ± SE of the number of mice per group. These results are representative of three similar experiments.
Th1-mediated antiviral protection requires IFN-γ responsiveness by the stromal, but not the hematopoietic, compartment
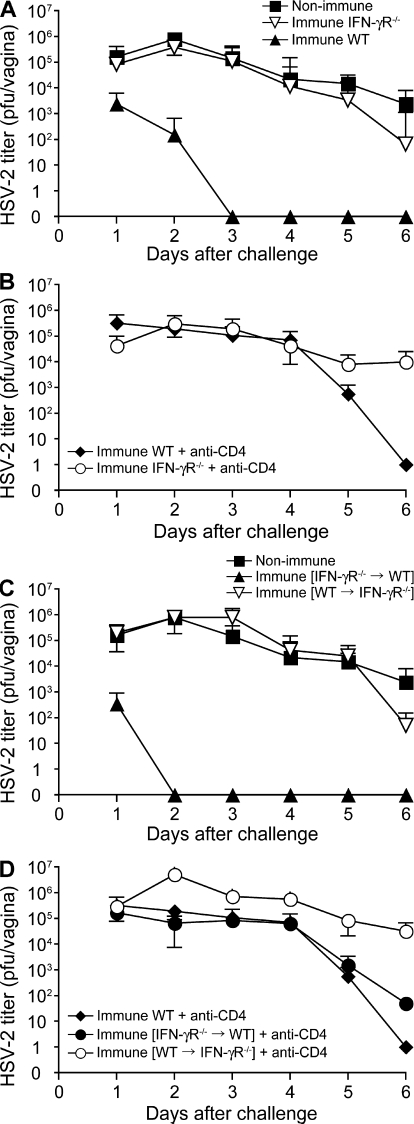
Having excluded direct cytolysis by perforin and FasL as the mechanism of Th1-mediated protection, we focused on the noncytolytic mechanism of control of viral replication. First, to examine whether IFN-γ activity is required for Th1-mediated protection, we analyzed the ability of the immunized IFN-γR−/−mice to control viral replication upon secondary challenge with WT HSV-2. Despite the normal development of memory Th1 responses (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20082039/DC1), immunized IFN-γR−/− mice were unable to control HSV-2 replication (Fig. 3 A). In contrast, immunized IFN-αβR−/− mice quickly cleared the HSV-2 virus and were mostly protected from disease (Fig. S4). These data showed that the responsiveness to IFN-γ is necessary for Th1-mediated protection against HSV-2 mucosal infection.
Figure 3.
Virus clearance mediated by memory CD4 T cells requires IFN-γ responsiveness by the stromal, but not the hematopoietic, compartment. 129 (WT; n = 9), IFN-γR−/− (n = 3), WT→IFN-γR−/− (n = 6), and IFN-γR−/−→WT mice (n = 6) were immunized with TK−HSV-2 ivag and, 4 wk later, challenged with lethal WT HSV-2. As a control, nonimmunized 129svj mice (n = 6) were challenged with WT HSV-2 (A and C). CD4 T cells were depleted from the respective groups of mice before secondary challenge with WT HSV-2 (B and D). Each bar represents the mean ± SE of the number of mice per group. Viral titers in vaginal washes after secondary challenge were measured. These results are representative of two similar experiments.
Next, to examine the cell types in which IFN-γ responsiveness is required for Th1-mediated virus clearance, we generated two sets of BM chimeric mice: IFN-γR−/− BM into WT mice (IFN-γR−/−→WT) and WT BM into IFN-γR−/− mice (WT→IFN-γR−/−). These mice were immunized ivag with TK−HSV-2 and were challenged with WT HSV-2 3 wk later. Within 3 d of secondary challenge, WT mice completely cleared the virus, whereas the virus titers of IFN-γR−/− remained high, which was similar to the naive WT mice (Fig. 3 A). In the IFN-γR−/−→WT BM chimera, in which only the stromal cells were responsive to IFN-γ, HSV-2 virus was completely cleared. In contrast, in the WT→IFN-γR−/− BM chimera in which the stromal cells failed to respond to IFN-γ, HSV-2 titers remained remarkably high, which was similar to those of naive mice after primary challenge (Fig. 3 C). Protection seen in all groups was completely abrogated by the depletion of CD4 T cells at the memory phase (Fig. 3, B and D). These data indicated that responsiveness to IFN-γ by the stromal, but not hematopoietic, compartment is essential for Th1-mediated protection against HSV-2.
Rapid induction of IFN-γ–mediated MHC class II molecules on vaginal epithelial cells after HSV-2 secondary infection
Thus far, our data showed the importance of IFN-γ responsiveness by stromal cells in Th1-mediated protection. However, it is unclear whether the infected epithelial cells themselves can present viral antigens on MHC class II. Thus, we examined whether vaginal epithelial cells can be induced to express MHC class II and whether such expression depends on IFN-γ. In naive and immune mice, vaginal epithelial cells did not constitutively express MHC class II (Fig. S5, A and C, available at http://www.jem.org/cgi/content/full/jem.20082039/DC1). However, in immune WT mice, MHC class II was rapidly and robustly up-regulated on vaginal epithelial cells after secondary viral challenge (Fig. S5 D). To examine the IFN-γ dependence of the MHC class II expression, we extended the analysis to the vaginal tissues of reinfected immune IFN-γR−/− and WT→IFN-γR−/− chimeric mice. Both of these groups of mice (Fig. S5, E and F) failed to express MHC class II on the vaginal epithelial cells. Thus, the vaginal epithelial cells, the primary target of HSV-2 infection, can express MHC class II in an IFN-γ–dependent manner.
Secretion of IFN-γ by Th1 cells, but not NK cells, is sufficient for protection against HSV replication
Our in vivo findings indicated that direct IFN-γ responsiveness by the vaginal stromal cells is required for the blockade of virus replication in immunized hosts. Examination of the vaginal wash at the early stage of secondary challenge revealed the presence of nanogram per milliliter levels of IFN-γ (Fig. S6 A, available at http://www.jem.org/cgi/content/full/jem.20082039/DC1). In primary keratinocytes, recombinant IFN-γ provided comparable antiviral protection as seen with IFN-α (Fig. S6 D). Less than 625 pg/ml of IFN-γ was found to be sufficient to completely prevent replication of HSV (Fig. S6 C). NK cells did not contribute to IFN-γ levels in the vaginal mucosa during secondary infection (Fig. S6 A). In contrast, rapid induction of IFN-γ (day 1) after secondary challenge was abrogated by anti-CD4 Ab injection (Fig. S6 A). Depletion of both CD4 and NK cells resulted in the complete blockade of IFN-γ. Collectively, these data suggested that in the absence of CD4 T cells, mice sustain high levels of viral load in the vagina (Fig. 1 F), leading to NK cell stimulation and IFN-γ secretion 2 d after rechallenge. To examine the relative abilities of the CD4 and NK cells to provide protection, we measured local viral titers in these mice. As shown previously, depletion of CD4 T cells abrogated antiviral protection in immune host (Fig. S6 B). In contrast, NK cells were not required for protection. These data demonstrated that in the absence of CD4 T cells, NK-secreted IFN-γ was unable to control viral replication. These data indicated that local Th1 cells rapidly recognize the virus and secrete IFN-γ within hours of infection (20), which is critical to providing protection. In contrast, NK cells failed to confer protection, suggesting that IFN-γ secreted in the vagina 2 d after reinfection is not sufficient for virus clearance. To directly test the hypothesis that the timing of IFN-γ secretion in the vagina is crucial in suppression of viral replication, we inoculated naive mice ivag with recombinant IFN-γ at either 2 or 48 h after infection with WT HSV-2 to mimic IFN-γ secretion by memory CD4 T cells (in immune mice) or NK cells (in naive mice), respectively (Fig. S7). Inoculation of IFN-γ at 2 h suppressed viral replication (Fig. S7 A), delayed pathology (Fig. S7 B), and reduced mortality (Fig. S7 C). In contrast, treatment with IFN-γ at 48 h after infection resulted in no enhancement in protection from HSV-2 infection. These data indicated that an early timing of IFN-γ secretion is critical in antiviral defense and suggest this as a mechanism for protection offered by memory CD4 T cells but not NK cells.
Direct recognition of viral antigen presented on MHC class II by the infected epithelial cells is not required for Th1-mediated protection
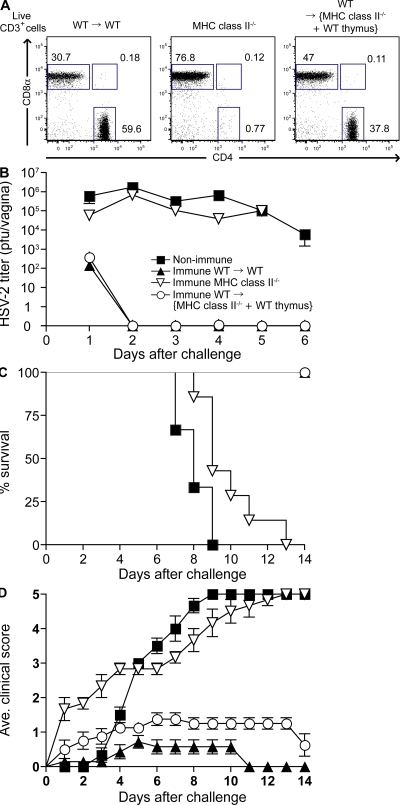
Our data indicated that protection against HSV-2 is dependent on CD4 T cells, independent of cytolysis, and requires IFN-γ responsiveness by stromal cells. The target of viral infection, the vaginal keratinocytes, responds to IFN-γ and up-regulates MHC class II expression. Thus, the most parsimonious explanation is that IFN-γ secreted by the Th1 cells provides protection. Next, we asked whether Th1 cells require direct recognition of the viral antigenic peptide presented by the MHC class II of the infected vaginal epithelial cells to mediate this effect. To this end, we generated BM chimeric mice that were capable of expressing MHC class II only by the hematopoietic cells. To preserve normal CD4 T cell repertoire in such mice, we first transplanted WT thymus under the kidney capsule of MHC class II–deficient mice to provide a source for thymic epithelial cells for positive selection of CD4 T cells. These mice were subsequently lethally irradiated and reconstituted with WT BM cells (WT→{MHC II−/− + WT thymus}). Upon reconstitution (>95% reconstituted after 8 wk [reference 26]), analysis of the peripheral T cells revealed normal CD4+ and CD8+ T cell development in the WT→{MHC II−/− + WT thymus} mice (Fig. 4 A) but, as expected, CD4 T cells failed to develop in WT→MHC II−/− mice without the thymic transplantation (Fig. S8, available at http://www.jem.org/cgi/content/full/jem.20082039/DC1). Using these mice, we examined whether antigen presentation by MHC class II on vaginal epithelial cells is critical for the Th1-mediated protection against lethal WT virus challenge. In contrast to the immune MHC II−/−mice and WT→MHC II−/− mice (Fig. S8), immune WT→{MHC II−/− + WT thymus} mice rapidly and completely eliminated the virus at the site of infection and were protected from disease upon secondary challenge with WT HSV-2 (Fig. 4, B–D). These data indicated that viral antigen presentation on MHC class II molecules by the infected vaginal epithelial cells is dispensable for the Th1-mediated viral clearance. These data also demonstrated that the recall activation of memory Th1 cells could be induced by the hematopoietic cells, most likely by MHC class II+ professional APCs at the mucosal surface.
Figure 4.
Direct recognition of the infected vaginal epithelial cells by Th1 cells is not required for protection. C57BL/6 (WT; n = 7), MHC class II−/− (n = 4), WT thymus-transplanted MHC class II−/− BM chimera, and WT→{MHC II−/− + WT thymus} (n = 9) were immunized with TK−HSV-2 ivag and, 4 wk later, challenged with lethal WT HSV-2. As a control, nonimmunized C57/BL6 mice (n = 6) were challenged with the virus. (A) FACS analyses of CD4 and CD8 T cells (live CD3+ cells) in the peripheral blood of C57BL/6 (WT)→WT, MHC class II−/−, and WT→{MHC II−/−+ WT thymus} mice. (B–D) Viral titers in vaginal washes (B), survival (C), and genital mean pathology scores (D) after secondary challenge were examined. Error bars represent the mean ± SE of the number of mice per group. These data are representative of three similar experiments.
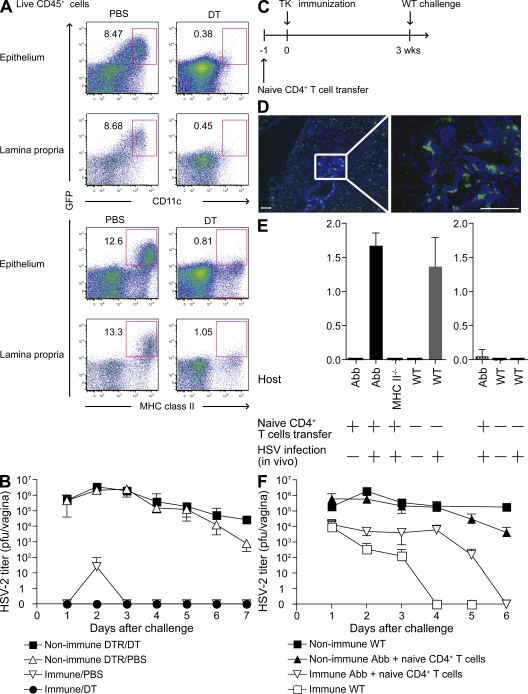
Conventional DCs are not required but are partially sufficient to induce recall Th1 response
Conventional DCs have been shown to be important in initiating recall CTL response in the LN, draining the sites of secondary infection in response to vesicular stomatitis virus, Listeria monocytogenes, and influenza virus infections (27). To examine whether conventional DCs are required to induce recall Th1 responses in the vagina, we generated CD11c–diphtheria toxin (DT) receptor (DTR) Tg BM→WT chimeric mice (27). Unlike the skin Langerhans cells, the vaginal epithelial DCs, along with the submucosal DCs, were reconstituted with the donor BM cells (26). Upon reconstitution, these mice were immunized with TK−HSV-2 and, 3 wk later, depleted of CD11c+ DCs by DT inoculation (Fig. S9, available at http://www.jem.org/cgi/content/full/jem.20082039/DC1). The DT injection regimen led to a near complete depletion of DCs from every organ examined, including the vaginal epithelium and lamina propria (Fig. 5 A), without affecting the CD11clo T cell populations owing to the low levels of CD11c expression by T cells compared with DCs (Fig. S9 E). Of note, non-DC MHC class II+ APCs were still present in the DT-treated CD11c-DTR Tg BM→WT chimeric mice (Fig. 5 A). These APCs consisted of both B cells and macrophages (Fig. S9 E). DC-depleted immune mice were rechallenged with WT HSV-2 ivag, Th1 cells were enumerated by ELISPOT assay (Fig. S10), and viral titers were measured from the vaginal washes (Fig. 5 B). These results indicated that the absence of DCs affected neither the number of IFN-γ–secreting cells in the vagina or spleen (Fig. S10) nor the ability of Th1 memory cells to mediate antiviral protection (Fig. 5 B).
Figure 5.
Conventional DCs are not required but are partially sufficient to elicit recall Th1 responses in the vagina. (A and B) TK−HSV-2-immunized CD11c-DTR→WT chimeras (n = 5) were inoculated with DT or PBS i.p., as shown in Fig. S9 (available at http://www.jem.org/cgi/content/full/jem.20082039/DC1). Depletion of vaginal DCs (CD11c+GFP+ and MHC II+GFP+) in the epithelium and lamina propria after DT injection was examined by FACS analysis (A). Viral titers in vaginal secretion were measured at the indicated days after challenge (B). (C–F) To examine the sufficiency of MHC class II expression on CD11c+ cells for protection against WT HSV-2 secondary challenge, Abb mice and MHC class II−/− mice were transplanted with naive polyclonal CD4 T cells (107 cells) 1 d before TK−HSV-2 immunization (C). MHC class II expression on vaginal DCs in TK−HSV-2–immunized mice (4 wk) was analyzed (D). Frozen sections were stained with MHC class II (green) and counterstained with DAPI (blue). Images were captured using a 10 (left) or 40× (right) objective. Bars, 100 μm. (E) Adoptively transferred naive CD4 T cells normally differentiate into Th1 cells in Abb mice immunized with TK−HSV-2. CD4 T cells isolated from the draining LNs of Abb, MHC class II−/− (MHCII−/−), and C57BL6 mice (WT) mice immunized ivag with TK−HSV-2 (7 d after infection) were cocultured with syngeneic splenocytes in the presence of HSV-2 antigens (left) or mock antigen (right) and analyzed for IFN-γ secretion (in nanograms/milliliter). Each column represents the mean ± SD of triplicate samples. (F) 3 wk after TK− immunization, Abb and MHC class II−/− mice were challenged with 104 PFU WT HSV-2 virus. Viral titers in vaginal secretion were measured at the indicated days after challenge. Error bars represent the mean ± SD. These data are representative of two similar experiments.
Next, we assessed whether DCs alone are sufficient to restimulate memory CD4 T cell response. To this end, we used the CD11c/Aβb (Abb) mouse model in which only CD11c+ DCs express MHC II (28). Because these mice do not develop CD4 T cells, naive CD4 T cells were adoptively transferred before immunization (Fig. 5 C). Unlike the skin Langerhans cells, which do not express sufficient CD11c and thus remain MHC II− in Abb mice (28), both the vaginal epithelial and submucosal DCs expressed MHC II in Abb mice (Fig. 5 D) owing to higher expression of CD11c (26). Thus, the use of Abb mice allowed us to examine whether vaginal epithelial and submucosal DCs are sufficient to induce recall response from memory Th1 cells. As reported previously (19), these mice developed normal Th1 response upon primary immunization with TK−HSV-2 (Fig. 5 E).
However, despite the presence of robust Th1, Abb mice had a significantly delayed clearance of viral load in the vagina compared with WT immune mice in which non-DCs also expressed MHC II (Fig. 5 F). Thus, collectively, our data indicated that the recall response of Th1 memory does not require presentation by either keratinocytes or DCs and that DCs alone are only partially capable of limiting viral replication. These results suggested that non-DC local MHC class II+ APCs must cooperate with DCs to induce recall stimulation of memory CD4 T cell response.
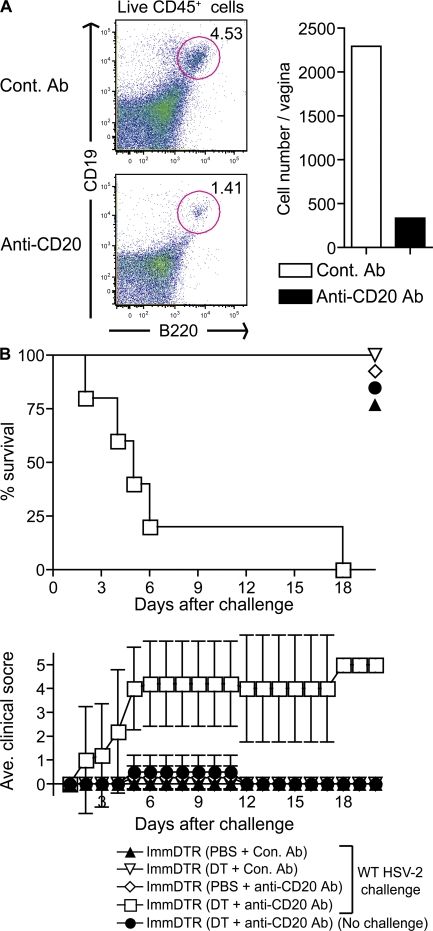
DCs and B cells maximize memory CD4 T cell response and provide protection
Finally, we examined the APC populations responsible for restimulating Th1 memory during secondary infection with HSV-2. To determine which MHC II+ cells have the potential to stimulate Th1 cells locally, we analyzed the MHC II+ populations from the vaginae of TK−HSV-2–immunized mice at 3 wk after infection. In addition to DCs, we detected both B cells and macrophages in the vaginal tissue of HSV-2–primed mice (Fig. S9, F and G). Based on these findings, we asked if DCs and macrophages or DCs and B cells cooperate to activate memory Th1 cells at the site of infection. To this end, we first depleted phagocytes using Clodronate liposome in HSV-immune mice before secondary challenge. However, despite the significant reduction in both the macrophages and DC numbers in the vaginal tissue, this treatment did not result in the loss of protection upon HSV-2 challenge (Fig. S11). Next, we depleted both DCs and B cells in HSV-2–immunized CD11c-DTR chimeric mice by treatment with DT (to deplete DCs) combined with injection of anti-CD20 Ab to deplete B cells (29). These treatments resulted in a significant reduction of the number of both DCs and B cells (Fig. 6 A and Fig. S9). Mice depleted of either DCs or B cells alone were completely protected from secondary HSV-2 challenge. However, importantly, depletion of DCs and B cells from immune mice abrogated Th1-mediated protection (Fig. 6 B). Because epithelial cells, and not APCs, were the only cells productively infected by HSV-2 (Fig. S12), these data suggested that uninfected DCs and B cells acquire viral antigens from infected epithelial cells and present them to local virus specific CD4 T cells. Collectively, these results demonstrated that DCs and B cells are both required to maximizing Th1 memory responses in vivo.
Figure 6.
DCs and B cells mediate memory Th1 recall response in the vagina. In addition to depletion of DCs, TK−HSV-2–immunized CD11c-DTR→WT chimeras (n = 5) were inoculated with either a control Ab or anti–mouse CD20 i.v., as shown in Fig. S9 (available at http://www.jem.org/cgi/content/full/jem.20082039/DC1). B cell depletion in the vaginal tissue of TK−HSV-2–immunized mice (3 wk after infection) after Ab injection was determined by FACS analysis (A). The mortality and mean clinical score are shown in B. Error bars represent the mean ± SD of five mice per group. These data are representative of three similar experiments.
DISCUSSION
Our results provide evidence for a mechanism by which memory Th1 cells mediate antiviral activity through secretion of IFN-γ after activation by local tissue-resident APCs (Fig. S13, available at http://www.jem.org/cgi/content/full/jem.20082039/DC1). In the case of genital herpes infection, the effector function of memory Th1 cells did not involve direct cytolysis by FasL or perforin. Furthermore, Th1 memory response did not require direct recognition of the infected vaginal epithelial cells via viral antigens presented on MHC class II. Instead, the main effector function of the memory Th1 cells was mediated by IFN-γ, which acted on the stromal cells of the vagina, particularly on the vaginal epithelial cells. In contrast, activation of hematopoietic cells by IFN-γ was dispensable for Th1-mediated protection. Finally, although IFN-γ secretion by memory Th1 cells in the vagina was elicited by hematopoietic cells, conventional DCs were neither required nor sufficient to fully recall responses at the local tissue. Instead, both DCs and B cells presented viral antigens to Th1 cells in the vaginal mucosa and restimulated memory Th1 cells during secondary viral challenge. These data revealed an important mechanism by which memory Th1 cells are elicited by tissue-resident noninfected APCs to provide disseminated antiviral protection through IFN-γ.
Currently, the known antiviral mechanisms of Th1 cells include providing help to sustain CTL and B cells, direct lysis of virally infected cells, and a nonlytic mechanism mediated by cytokines (10). Our study revealed an important mechanism by which the Th1 cells are elicited to induce IFN-γ to clear and prevent viral spread, a process which is independent of direct recognition of infected cells but involves antigen presentation by tissue-resident professional APCs. This pathway of Th1 recall has several advantages over the other mechanisms. First, this mode of restimulation cannot be inhibited by virally encoded genes that prevent antigen presentation on MHC class II in the infected cells. Second, the antiviral effects can be quickly disseminated to a wide range of target cells in a given tissue, allowing rapid and complete antiviral state to be established in the susceptible mucosa. Third, because of the nonlytic nature of the protection, tissue injury and viral spread caused by the release of virions from lysed cells can be avoided. Finally, this mode of Th1 effector function represents an effective means of antiviral protection against viruses that do not infect MHC class II+ APCs. In fact, several previous studies have implicated that Th1-mediated antiviral protection can occur indirectly for viruses that infect MHC class II− target cells (13, 30, 31). Because only a handful of viruses actively target and replicate in professional APCs, such means of protection can be exploited for vaccine purposes.
Our data revealed that IFN-γ responsiveness by the hematopoietic cells was not required for protection. A similar observation was reported recently for CD8-mediated protection against HSV infection (31). More recently, effector CD4 T cells were shown to be involved in clearance of HSV-1 from the neuronal tissues in a manner independent of Fas or perforin (32). Our results ruled out the need for DCs, macrophages, γδ T cells, NK cells, NK T cells, or Th1 cells themselves to be restimulated through the IFN-γR to elicit or mediate the recall responses. Because macrophage activation by IFN-γ plays a critical role in antibacterial and antiprotozoan responses, these findings highlight the different mode of action by which Th1 cells provide immunity to diverse sets of pathogens. In addition, these results imply that IFN-γ–mediated up-regulation of MHC class II genes on tissue-resident APCs is also not required for the induction of local recall Th1 responses and suggest that recall response is sufficiently mediated by non-DC APCs that have constitutive expression of MHC class II. It is unclear what IFN-γ–induced genes are responsible for the suppression of viral replication in the vaginal epithelial cells. At the innate stage, antiviral protection conferred by type I IFNs is in part mediated by protein kinase R (33) but not the RNaseL (34) pathways. Identification of the IFN-γ–inducible effectors that can suppress HSV-2 replication will be an important future endeavor. In hepatitis B virus Tg mice, transferred virus-specific Th1 cells secreted antiviral cytokines by recognizing antigen presented by nonparenchymal cells (10). However, the type or origin of such APCs remains unknown. Our data demonstrated that local DCs and B cells stimulate memory Th1 cells during secondary viral challenge. This is in contrast to the selective requirement for DCs in memory CD8 T cell activation (27) and highlights the distinct cellular subsets involved in memory CD8 versus CD4 T cell recall responses. B cells and DCs have been shown to contribute to priming of CD4 T cell response during L. monocytogenes infection (35). It is interesting to speculate that because of the ability of B cells to selectively take up viral antigens through their B cell receptors, these cells may become potent APCs and provide cognate help to memory Th1 cells. In addition, because of the antigen specificity provided by memory B cells, they may constitute “memory APCs.” Future studies to determine whether memory B cells provide help to T helper cells during secondary infection through BCR-specific uptake of antigens will likely yield new insights.
In summary, our results provide evidence for an important mechanism by which memory Th1 cells mediate antiviral protection. Because the memory Th1 cells did not require direct recognition of viral antigens presented by the infected cells via MHC class II, particularly for viruses that encode evasion molecules to avoid recognition by the adaptive immune cells, eliciting Th1 memory response might represent an ideal vaccine approach. In addition, depending on the mode of protection, mobilizing local DCs and B cells to restimulate memory CD4 T cells may be beneficial in eliminating viral pathogens in chronically infected host.
MATERIALS AND METHODS
Mice.
6–8-wk-old female C57BL/6 (CD45.2+), congenic C57BL6 mice, B6.SJL-PtprcaPep3b/BoyJ (B6.Ly5.1; CD45.1+), and 129/svJ were purchased from the National Cancer Institute and the Jackson Laboratory. B6.129-H2dlAb1-Ea/J (MHC class II−/−), B6.MRL-Faslpr/J (B6.MRL/lpr), C57BL/6-Prf1tm1Sdz/J (Perforin−/−), and CD11c-DTR Tg mice (B6.FVB-Tg [Itgax-DTR/GFP] 57Lan/J) were obtained from the Jackson Laboratory. IFN-γR−/− and IFN-αβR−/− mice (36) on a 129S2 background were provided by H.W. Virgin IV (Washington University, St. Louis, MO). CD11c/Aβb (Abb) mice have been described previously (28). All procedures used in this study complied with federal guidelines and institutional policies by the Yale animal care and use committee.
Viruses.
186syn−TK− (37) and 186syn+ (38) were gifts of D. Knipe (Harvard Medical School, Boston, MA). HSV-1 KOS VP26-GFP (HSV-1/GFP) (39) was a gift from P. Desai and S. Person (John's Hopkins University, Baltimore, MD). HSV-2 was propagated and titered on Vero cells as previously described (19). Influenza virus A/PuertoRico/3/334 (A/PR8; H1N1) was a gift from H. Hasegawa (National Institute of Infectious Diseases, Tokyo, Japan) and was propagated in 10-d fertile chicken eggs and titered on the Madin-Darby canine kidney cells (40).
Antibodies.
Anti-CD3ε (145-2C11), anti-CD45.2 (104), anti-CD45.1 (A20), anti-CD4 (GK1.5 and RM4-4), and anti–MHC class II (M5/114.15.2) were purchased from eBioscience. Anti-CD8α (53–6.7) and anti-CD45R/B220 (RA3-6B2) were purchased from BD. Anti-CD68 (FA-11) was obtained from AbD Serotec.
Flow cytometry.
The DCs and lymphocytes from vaginal epithelia and lamina propria were isolated and analyzed as previously described (26) (Supplemental materials and methods, available at http://www.jem.org/cgi/content/full/jem.20082039/DC1). Single-cell suspensions from spleen, draining LNs (inguinal LN and iliac LNs), and vagina were pretreated with anti-Fc receptor Ab (eBioscience) at 4°C for 10 min. These cells were stained with various antibodies at 4°C for 15 min. 7-amino-actinomycin d (7-AAD; BD) was added to the cells immediately before analysis and 7-AAD+ dead cells were excluded from analysis. Multiparameter analyses were performed on a flow cytometer (LSR II; BD) and were analyzed using FlowJo software (Tree Star, Inc.).
Construction of BM chimeric mice.
BM chimeric mice using C57BL/6, MHC class II−/−, B6.MRL/lpr, and Perforin−/− mice were constructed by a standard method (26). Irradiated recipient mice were reconstituted with 5 × 106 cells of the appropriate cell suspension by means of i.v. injection. To generate BM chimeric mice using 129/svJ and IFN-γR−/− mice, the recipient mice were irradiated with 925 rads and then reconstituted with 1 × 107 cells from BM as previously reported (41). The reconstituted mice were maintained in a clean facility for at least 8 wk before use.
Immunization and viral challenge.
Female mice were injected s.c. in the neck ruff with progesterone (Depo Provera; GE Healthcare) at 2 mg per mouse in a 100-μl volume. These mice were immunized ivag with 106 PFU HSV-2 (186syn− and TK−) in a 10-μl volume. At 3–4 wk later, immunized mice were challenged with 104 PFU HSV-2 (186syn+) ivag using a previously described protocol (5). The severity of disease was scored as follows (42): 0, no sign; 1, slight genital erythema and edema; 2, moderate genital inflammation; 3, purulent genital lesions; 4, hindlimb paralysis; and 5, premoribund. Because of humane concerns, the animals were killed before reaching the moribund state.
DT-mediated DC depletion.
For systemic DC depletion in vivo, CD11c-DTR→WT BM chimeras were inoculated ivag with 320 ng DT (Sigma-Aldrich) daily between days −6 and −1 by i.p. injection of WT virus challenge. Depletion of CD11c+ cells was assessed by FACS of splenocytes and vaginal leukocytes.
In vivo depletion of lymphocytes.
Depletion of CD4 or CD8 T cells were performed at days −5, −3, and −1 and 1, 3, and 5 after rechallenge by i.p injection of 400 μg of anti-CD4 (GK1.5; rat IgG2b) or anti-CD8 (53–6.72; rat IgG2a) Abs. Control mice were injected with 400 μg of rat IgG. In vivo depletion was confirmed by FACS analysis of the cell suspension from secondary lymphoid organs and vaginae of the Ab-treated mice at 4 d after secondary challenge. For NK cell depletion, purified anti–mouse NK1.1 (PK136; eBioscience) was given an i.p. injection of 200 μg Ab in PBS 2 d before and 1 d after challenge as previously reported (43). For B cell depletion, 250 μg of purified anti–mouse CD20 (18B12; mouse IgG2a) or anti–human CD20 Ab (2B8; mouse IgG2a) in PBS was given via i.v. injection at 4 and 8 d before challenge (29). For phagocyte depletion in blood and vagina, clodronate liposome or PBS liposome (250 μl for i.v. and 10 μl for ivag) were injected at 4 and 1 d before challenge. These liposomes were a gift from Roche and were generated as previously described (44).
Vaginal viral titers and cytokine measurement.
Vaginal fluids were collected on days 1–7 after infection using calcium alginate swabs and PBS. Viral titers were obtained by titration of vaginal wash samples on Vero cell monolayer as described previously (19). IFN-α and IFN-γ were detected in vaginal fluids by ELISA as previously described (45).
Adoptive transfer of naive or effector T cells.
For preparation of naive CD4, effector CD4, and effector CD8 T cells for adoptive transfer, congenic C57BL/6 (CD45.1+) mice were inoculated ivag with mock, TK−HSV-2 (1 × 106 PFU/mouse), or influenza virus A/PR8 (3 × 105 PFU/mouse) for 7 d (46). CD4 and CD8 T cells were purified using a MACS column (Miltenyi Biotec). The selected CD4+ or CD8+ T cells (>90% purity) were used for adoptive transfer. Six to ten million purified cells were transferred i.v. into the CD45.2+ C57BL/6 recipient. 2 h after transfer, these recipients were challenged with 104 PFU WT HSV-2 virus or 1 × 106 PFU of influenza virus A/PR/8. Vaginae were harvested at 3 d after virus infection and were processed and analyzed by immunofluorescence staining as described in Immunofluorescence staining.
Thymic transplantation.
The thoracic cavity of neonatal B6 pups (<2 d old) was exposed, and thymic lobes were removed and placed in PBS. Neonatal syngeneic mouse thymus was grafted under the right kidney capsule of MHC class II−/− mice. 2 wk after transplantation, these recipients were irradiated and reconstituted with WT BM cells as described previously (26). To confirm CD4 T cell development, 8 wk after BM transplantation, lymphocytes were collected from the peripheral blood, and the percentages of CD4 and CD8 T cell populations of these mice were assessed by flow cytometry.
Immunofluorescence staining.
To examine the localization of CD4, CD8, and MHC class II–expressing cells, frozen sections of vaginae collected from various experimental groups were stained with the Abs (anti-CD4 [L3T4 and H129.19], anti–CD8-α [53–6.7], or anti–MHC class II) in a previously described procedure (19). Likewise, HSV-2 and CD45.1 antigens were detected using biotin-conjugated anti–mouse CD45.1 Ab and FITC-conjugated mAbs anti-HSV 1 and 2, respectively, which are specific for envelope glycoprotein (ViroStat). Stained slides were washed and incubated with DAPI and mounted with Fluoromount-G (SouthernBiotech). These slides were analyzed by fluorescence microscopy (BX51; Olympus).
Online supplemental material.
Fig. S1 shows the antigen specificity of migration of Th1 cells after viral challenge into the vaginal tissue. Fig. S2 shows the depletion of CD4+ T cells, but not APCs, upon anti-CD4 Ab treatment. Fig. S3 shows Th1 response in draining LNs in WT or IFN-γR−/− mice after TK− immunization for 3 wk. Fig. S4 shows HSV-2 titer and clinical score of WT and IFN-αβR−/− mice after HSV-2 secondary challenge. Fig. S5 shows up-regulation of MHC class II mediated by IFN-γ signaling on vaginal epithelial cells after secondary HSV-2 challenge. Fig. S6 shows IFN-γ secretion in vaginal wash of TK−HSV-2–immunized mice after secondary challenge, treated with anti-CD4 Ab and/or anti-NK1.1 Ab, and the effect of IFN-γ or IFN-α on the protection of primary keratinocytes against in vitro HSV-2 infection. Fig. S7 shows the effectiveness of topically applied recombinant IFN-γ in preventing viral replication and viral disease after lethal WT HSV-2 challenge. Fig. S8 shows a lack of protection in TK−HSV-2–immunized WT into MHC class II−/− BM chimera after secondary challenge. Fig. S9 shows DT injection scheme and the efficacy of CD11c+ cell and B cell depletion, but not memory Th1 cells, in vagina, spleen, and draining LNs. It also demonstrates APC populations in the vagina of HSV-2–primed mice. Fig. S10 shows the enumeration of HSV-2–specific Th1 response in vagina of TK−HSV-2–immunized mice with or without DC depletion. Fig. S11 shows HSV-2–infected cells in the vagina of nonimmune mice. Fig. S12 shows that HSV-2 predominantly infects epithelial cells and not APCs. Fig. S13 shows a schematic representation of memory Th1 cell–mediated antiviral immunity in mucosal tissues. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082039/DC1.
Supplementary Material
Acknowledgments
We are grateful for Dr. T. Ichinohe for help with influenza virus infection.
This study was supported by grants from the National Institutes of Health (AI054359 and AI064705). N. Iijima was a recipient of a postdoctoral fellowship from the Japan Antibiotics Research Association Pfizer Infectious Diseases Research Fund. A. Iwasaki holds an Investigator in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
The authors have no conflicting financial interests.
Abbreviations used: Ab, antibody; DN, double negative; DT, diphtheria toxin; DTR, DT receptor; ivag, intravaginal; Tg, transgenic; TK, thymidine kinase.
References
- 1.Bachmann, M.F., and R.M. Zinkernagel. 1997. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15:235–270. [DOI] [PubMed] [Google Scholar]
- 2.Burton, D.R., R.L. Stanfield, and I.A. Wilson. 2005. Antibody vs. HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. USA. 102:14943–14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen, J.P., R.D. Cardin, K.C. Branum, and P.C. Doherty. 1999. CD4(+) T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc. Natl. Acad. Sci. USA. 96:5135–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harandi, A.M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Differential roles of B cells and IFN-gamma-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 82:845–853. [DOI] [PubMed] [Google Scholar]
- 5.Milligan, G.N., D.I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093–6100. [PubMed] [Google Scholar]
- 6.Kuklin, N.A., M. Daheshia, S. Chun, and B.T. Rouse. 1998. Role of mucosal immunity in herpes simplex virus infection. J. Immunol. 160:5998–6003. [PubMed] [Google Scholar]
- 7.Parr, M.B., and E.L. Parr. 1998. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 72:2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn, J.L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129. [DOI] [PubMed] [Google Scholar]
- 9.Gollob, K.J., L.R. Antonelli, and W.O. Dutra. 2005. Insights into CD4+ memory T cells following Leishmania infection. Trends Parasitol. 21:347–350. [DOI] [PubMed] [Google Scholar]
- 10.Franco, A., L.G. Guidotti, M.V. Hobbs, V. Pasquetto, and F.V. Chisari. 1997. Pathogenetic effector function of CD4-positive T helper 1 cells in hepatitis B virus transgenic mice. J. Immunol. 159:2001–2008. [PubMed] [Google Scholar]
- 11.Guidotti, L.G., and F.V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65–91. [DOI] [PubMed] [Google Scholar]
- 12.Sitati, E.M., and M.S. Diamond. 2006. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J. Virol. 80:12060–12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherle, P.A., G. Palladino, and W. Gerhard. 1992. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J. Immunol. 148:212–217. [PubMed] [Google Scholar]
- 14.Brown, D.M., A.M. Dilzer, D.L. Meents, and S.L. Swain. 2006. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J. Immunol. 177:2888–2898. [DOI] [PubMed] [Google Scholar]
- 15.Landais, E., X. Saulquin, M. Bonneville, and E. Houssaint. 2005. Long-term MHC class II presentation of the EBV lytic protein BHRF1 by EBV latently infected b cells following capture of BHRF1 antigen. J. Immunol. 175:7939–7946. [DOI] [PubMed] [Google Scholar]
- 16.Maloy, K.J., C. Burkhart, T.M. Junt, B. Odermatt, A. Oxenius, L. Piali, R.M. Zinkernagel, and H. Hengartner. 2000. CD4+ T cell subsets during virus infection: protective capacity depends on effector cytokine secretion and on migratory capability. J. Exp. Med. 191:2159–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milligan, G.N., and D.I. Bernstein. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 229:259–268. [DOI] [PubMed] [Google Scholar]
- 18.Milligan, G.N., K.L. Dudley-McClain, C.G. Young, and C.F. Chu. 2004. T-cell-mediated mechanisms involved in resolution of genital herpes simplex virus type 2 (HSV-2) infection of mice. J. Reprod. Immunol. 61:115–127. [DOI] [PubMed] [Google Scholar]
- 19.Zhao, X., E. Deak, K. Soderberg, M. Linehan, D. Spezzano, J. Zhu, D.M. Knipe, and A. Iwasaki. 2003. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parr, M.B., and E.L. Parr. 1999. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 258:282–294. [DOI] [PubMed] [Google Scholar]
- 21.Neumann, J., A.M. Eis-Hubinger, and N. Koch. 2003. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J. Immunol. 171:3075–3083. [DOI] [PubMed] [Google Scholar]
- 22.Yanai, F., E. Ishii, K. Kojima, A. Hasegawa, T. Azuma, S. Hirose, N. Suga, A. Mitsudome, M. Zaitsu, Y. Ishida, et al. 2003. Essential roles of perforin in antigen-specific cytotoxicity mediated by human CD4+ T lymphocytes: analysis using the combination of hereditary perforin-deficient effector cells and Fas-deficient target cells. J. Immunol. 170:2205–2213. [DOI] [PubMed] [Google Scholar]
- 23.Dobbs, M.E., J.E. Strasser, C.F. Chu, C. Chalk, and G.N. Milligan. 2005. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin-or Fas-mediated cytolytic mechanisms. J. Virol. 79:14546–14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe-Fukunaga, R., C.I. Brannan, N.G. Copeland, N.A. Jenkins, and S. Nagata. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 356:314–317. [DOI] [PubMed] [Google Scholar]
- 25.Kim, S., K.A. Kim, D.Y. Hwang, T.H. Lee, N. Kayagaki, H. Yagita, and M.S. Lee. 2000. Inhibition of autoimmune diabetes by Fas ligand: The paradox is solved. J. Immunol. 164:2931. [DOI] [PubMed] [Google Scholar]
- 26.Iijima, N., M.M. Linehan, S. Saeland, and A. Iwasaki. 2007. Vaginal epithelial dendritic cells renew from bone marrow precursors. Proc. Natl. Acad. Sci. USA. 104:19061–19066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zammit, D.J., L.S. Cauley, Q.M. Pham, and L. Lefrancois. 2005. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 22:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemos, M.P., L. Fan, D. Lo, and T.M. Laufer. 2003. CD8alpha+ and CD11b+ dendritic cell-restricted MHC class II controls Th1 CD4+ T cell immunity. J. Immunol. 171:5077–5084. [DOI] [PubMed] [Google Scholar]
- 29.Hamel, K., P. Doodes, Y. Cao, Y. Wang, J. Martinson, R. Dunn, M.R. Kehry, B. Farkas, and A. Finnegan. 2008. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J. Immunol. 180:4994–5003. [DOI] [PubMed] [Google Scholar]
- 30.Topham, D.J., and P.C. Doherty. 1998. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J. Virol. 72:882–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bird, M.D., C.F. Chu, A.J. Johnson, and G.N. Milligan. 2007. Early resolution of herpes simplex virus type 2 infection of the murine genital tract involves stimulation of genital parenchymal cells by gamma interferon. J. Virol. 81:423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson, A.J., C.F. Chu, and G.N. Milligan. 2008. Effector CD4+ T-cell involvement in clearance of infectious herpes simplex virus type 1 from sensory ganglia and spinal cords. J. Virol. 82:9678–9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr, D.J., T. Wuest, L. Tomanek, R.H. Silverman, and B.R. Williams. 2006. The lack of RNA-dependent protein kinase enhances susceptibility of mice to genital herpes simplex virus type 2 infection. Immunology. 118:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duerst, R.J., and L.A. Morrison. 2007. Herpes simplex virus type 2-mediated disease is reduced in mice lacking RNase L. Virology. 360:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouaziz, J.D., K. Yanaba, G.M. Venturi, Y. Wang, R.M. Tisch, J.C. Poe, and T.F. Tedder. 2007. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc. Natl. Acad. Sci. USA. 104:20878–20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller, U., U. Steinhoff, L.F. Reis, S. Hemmi, J. Pavlovic, R.M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science. 264:1918–1921. [DOI] [PubMed] [Google Scholar]
- 37.Coen, D.M., M. Kosz-Vnenchak, J.G. Jacobson, D.A. Leib, C.L. Bogard, P.A. Schaffer, K.L. Tyler, and D.M. Knipe. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA. 86:4736–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao, M., J. Bouchey, K. Curtin, and D.M. Knipe. 1988. Genetic identification of a portion of the herpes simplex virus ICP8 protein required for DNA-binding. Virology. 163:319–329. [DOI] [PubMed] [Google Scholar]
- 39.Desai, P., and S. Person. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72:7563–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichinohe, T., I. Watanabe, E. Tao, S. Ito, A. Kawaguchi, S. Tamura, H. Takahashi, H. Sawa, M. Moriyama, J. Chiba, K. Komase, Y. Suzuki, T. Kurata, T. Sata, and H. Hasegawa. 2006. Protection against influenza virus infection by intranasal vaccine with surf clam microparticles (SMP) as an adjuvant. J. Med. Virol. 78:954–963. [DOI] [PubMed] [Google Scholar]
- 41.Yap, G.S., and A. Sher. 1999. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-γ– and tumor necrosis factor (TNF)-α–dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 189:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison, L.A., X.J. Da Costa, and D.M. Knipe. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 243:178–187. [DOI] [PubMed] [Google Scholar]
- 43.Usherwood, E.J., S.K. Meadows, S.G. Crist, S.C. Bellfy, and C.L. Sentman. 2005. Control of murine gammaherpesvirus infection is independent of NK cells. Eur. J. Immunol. 35:2956–2961. [DOI] [PubMed] [Google Scholar]
- 44.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 174:83–93. [DOI] [PubMed] [Google Scholar]
- 45.Lund, J.M., M.M. Linehan, N. Iijima, and A. Iwasaki. 2006. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 177:7510–7514. [DOI] [PubMed] [Google Scholar]
- 46.Garulli, B., Y. Kawaoka, and M.R. Castrucci. 2004. Mucosal and systemic immune responses to a human immunodeficiency virus type 1 epitope induced upon vaginal infection with a recombinant influenza A virus. J. Virol. 78:1020–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.