SUMMARY AND CONCLUSIONS
In situ hybridization with subtype-specific probes was used to ask whether there is a change in the types of sodium channels that are expressed in dorsal root ganglion (DRG) neurons after axotomy.
Types I and II sodium channel mRNA are expressed at moderate-to-high levels in control DRG neurons of adult rat, but type III sodium channel mRNA is not detectable.
When adult rat DRG neurons are examined by in situ hybridization 7–9 days following axotomy, type III sodium channel mRNA is expressed at moderate-to-high levels, in addition to types I and II mRNA that are present at relatively high levels.
To determine whether the expression of type III sodium channel mRNA following axotomy represents up-regulation of a gene that had been expressed at earlier developmental stages, we also studied DRG neurons from embryonic (E17) rats. In these embryonic DRG neurons, type I sodium channel mRNA is expressed at low levels, type II mRNA at high levels, and type III at high levels.
These results demonstrate altered expression of sodium channel mRNA in DRG neurons following axotomy, and suggest that in at least some DRG neurons, there is a de-differentiation after axotomy that includes a reversion to an embryonic mode of sodium channel expression. Different channel characteristics, as well as an altered spatial distribution of sodium channels, may contribute to the electrophysiological changes that are observed in axotomized neurons.
INTRODUCTION
It is well known that, following axonal transection, there are retrograde changes in the neuronal cell body that reflect disconnection from post-synaptic targets (Foehring et al. 1987; Purves and Nja 1978), altered axo-glial interactions, (Bhisitkul et al. 1990) or an intrinsic response of the neuron to injury (Grafstein 1986; Waxman and Anderson 1982). The retrograde axon reaction following axotomy includes changes in sodium channel expression over the neuronal cell body and dendrites (see Titmus and Faber 1990 for review). These changes are reflected by altered excitability of the axon initial segment and soma-dendritic compartment (Eccles et al. 1958; Gallego et al. 1987; Kuno and Llinas 1970; Shapavolov and Grantyn 1968) and have been interpreted as being due to an altered distribution of voltage-sensitive sodium channels over the axon, cell body, and dendrites (Dodge and Cooley 1973; Sernagor et al. 1986; Titmus and Faber 1986). On the basis of the changes in sodium channel distribution and in other electrophysiological parameters, it has been suggested that axotomy may be followed by a de-differentiation of the injured neuron (Foehring et al. 1986; Kuno et al. 1974; Titmus and Faber 1990). However, the studies carried out to date have focused on the spatial distribution of sodium channels in axotomized neurons and have not examined the question of whether different types of sodium channels are synthesized by neurons before and after injury of the axon.
Spinal sensory neurons (dorsal root ganglion neurons, DRG neurons) are known to express several distinct voltage-sensitive sodium currents that can be differentiated on the basis of voltage-dependence of activation and inactivation, kinetics, and tetrodotoxin (TTX) sensitivity (Caffrey et al. 1992; Honmou et al. 1994; Kostyuk et al. 1981; McLean et al. 1988; Roy and Narahashi 1992; Yoshida et al. 1978). Developmental changes have been observed in the expression of these physiologically and pharmacologically different sodium currents in DRG neurons (Roy and Narahashi 1992; Schwartz et al. 1990), and exposure to nerve growth factor (NGF) has been noted to differentially promote the expression of a TTX-resistant sodium current in these cells (Omri and Meiri 1990).
Increased mRNA synthesis has been observed in sensory neurons following axotomy (Barron 1989; Langford et al. 1980; Wells 1987). We have recently developed in situ hybridization methods using subtype-specific riboprobes that permit the analysis of sodium channel mRNA expression in identified cells (Black et al. 1994a,b). In the present study we have used these methods to examine the expression in DRG neurons of mRNA for the type I, II, and III sodium channel α subunit (Kayano et al. 1988; Noda et al. 1986) and asked whether there are changes in sodium channel mRNA expression in these cells following axotomy. Our observations indicate that axotomy is followed by the expression of mRNA for a sodium channel α subunit (type III) that is not normally expressed by mature DRG neurons. To determine whether this altered mode of sodium channel mRNA production represents up-regulation following axonal injury of a gene that had been expressed at an earlier stage of development, we also examined sodium channel mRNA expression in embryonic DRG neurons.
MATERIALS AND METHODS
Probes
Plasmids pBSNC2 and pBSNC3 carrying 3′-non-coding regions of rat brain sodium channel cDNA II (Noda et al. 1986) and III (Kayano et al. 1988) were constructed as previously described (Black et al. 1994a,b); these regions corresponded to nucleotide sequences 6602-7078 and 5920-6399 (numbering sequence according to Noda et al. 1986 and Kayano et al. 1988) for sodium channel mRNA II and III, respectively. The amplified DNA fragment for sodium channel I (nucleotide sequence 7134-7569; Noda et al. 1986) was digested with Sau 3AI and A1uI and ligated to the EcoRV/BamHI fragment from pBSSK(-) to yield pBSNCI. Due to a possible point mutation at nucleotide 7231 (Noda et al. 1986) in the amplified DNA fragment, the insert for pBSNCI was 331 nucleotides in length (7231-7562). The inserts were sequenced by the dideoxy chain termination method, and were specific for sodium channel mRNA I, II, and III. Computer-assisted best fit searches for homology showed no specific homology between the sodium channel isoform-specific sequences or between these sequences and known GenEMBL sequences.
Digoxigenin-labeled, single-strand RNA probes were prepared using DIG RNA Labeling Kit (Boehringer Mannheim) according to the manufacturer’s instructions. For generation of anti-sense probes HindIII-cleaved pBSNC1, EcoRI-cleaved pBSNC2, and BamHI-cleaved pBSNC3 were used as templates for transcription with T3 or T7 RNA polymerase (Black et al. 1994a). The reaction products were fractionated by gel filtration (Sephadex G-50, Pharmacia).
Axotomy
Axotomy to adult dorsal root ganglion neurons was performed as previously described (Bhisitkul et al. 1990). Adult Wistar female rats were anesthetized with an injection of ketamine (40 mg/kg ip) and xylazine (2.5 mg/kg ip). The left sciatic nerve was exposed and a tight ligature with 4–0 silk suture was placed around the sciatic nerve near the sciatic notch proximal to the pyriform ligament. The nerve was sectioned immediately distal to the ligature site, the avulsed distal nerve was retracted and the wound was closed.
Tissue preparation/in situ hybridization
Adult axotomized (7–9 days postaxotomy) and timed-pregnant albino rats were deeply anesthetized with chloral hydrate and perfused through the heart, first with a phosphate-buffered saline (PBS) solution and then with 4% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer, pH 7.4, at 4°C. Following perfusion fixation, axotomized and nonoperated dorsal root ganglia at levels L4, 5, and 6 were collected and placed in fresh fixative at 4°C. Embryonic day 17 (E-17) fetuses were collected from pregnant rats and placed in fresh fixative at 4°C. After 2–4 h, the tissue was transferred to a solution containing 4% paraformaldehyde and 30% sucrose in 0.14 M phosphate buffer and stored overnight at 4°C. The vertebral column, including spinal cord and DRGs, of E-17 fetuses was carefully dissected, frozen, and 15 μm cryostat sections were mounted on poly-l-lysine-coated slides. Control and axotomized DRGs were frozen and 10 μm sections were cut and placed on poly-l-lysine-coated slides. The slides were processed for in situ hybridization cytochemistry as previously described (Black et al. 1994a), except the E-17 tissue was incubated in proteinase K solution for 15 min instead of 25 min. Following in situ hybridization cytochemistry, the slides were dehydrated, cleared and mounted with Permount.
RESULTS
The cellular distribution of sodium channel α-subunit mRNAs I, II, and III in control, axotomized and embryonic E-17 DRG was examined with nonisotope in situ hybridization methods, utilizing isoform-specific riboprobes from the 3′-noncoding (NCI, NCII, NCIII) regions of rat brain sodium channel mRNAs.
Control DRG
Sodium channel mRNA I expression in DRG neurons from adult rats was variable, ranging from negligible in some neurons to high levels in the majority of the neurons (Fig. 1B). In contrast, sodium channel mRNA II was expressed at moderate levels in virtually all DRG neurons (Fig. 1E). Sodium channel mRNA III was not detectable in control adult DRGs (Fig. 1H).
Fig. 1.
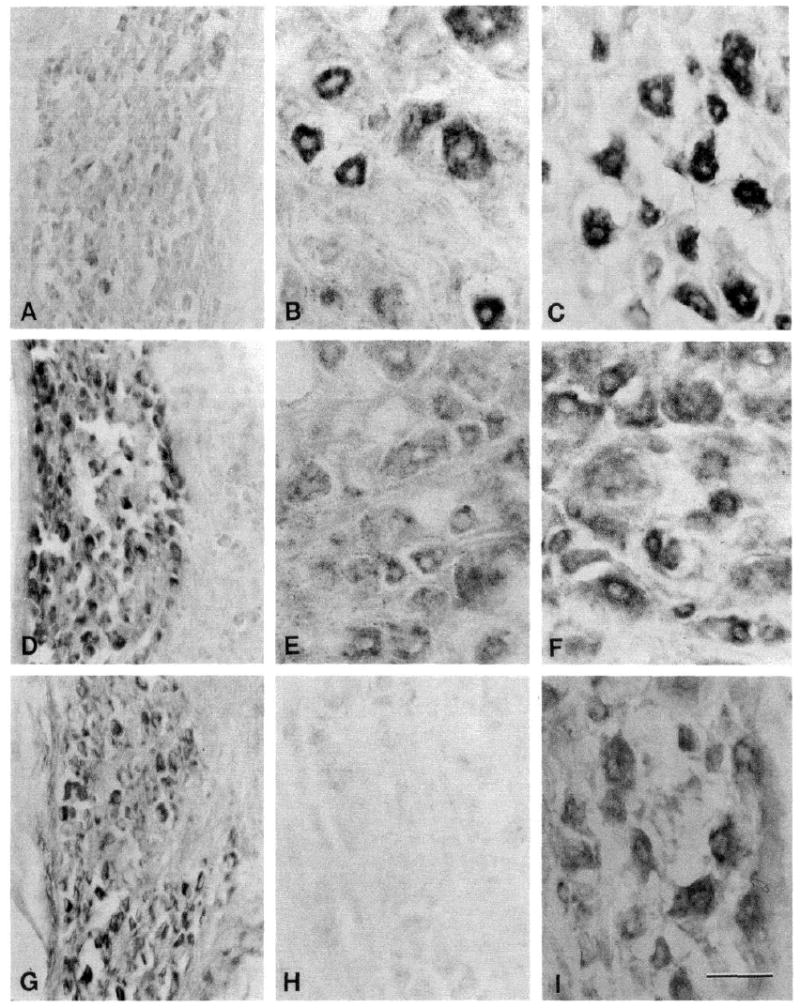
Sodium channel mRNAs in embryonic day 17 (E-17), adult and adult axotomized dorsal root ganglions (DRGs). DRGs from E-17 fetuses (A, D, and G), control adult (B, E, and H) and axotomized adult (C, F, and I) were probed for sodium channel mRNA I (A, B, and C), II (D, E, and F) and III (G, H, and I). Sodium channel mRNA I is generally expressed at low levels in E-17 DRGs (A) and at high levels in control (B) and axotomized (C) DRGs. Sodium channel mRNA II is expressed at moderate-to-high levels in E-17 DRGs (D) and generally at moderate levels in control (E) and axotomized (F) DRGs. Sodium channel mRNA III is not detectable in adult control DRGs (H), but is expressed at moderate-to-high levels in E-17 (G) and adult axotomized (I) DRGs. Scale bar, 50 μm.
Axotomized DRG
Dorsal root ganglion neurons were probed with isoform-specific sodium channel riboprobes NCI, NCII, and NCIII 7–9 days following axotomy. Sodium channel mRNA I was expressed by most DRG neurons, and these neurons generally displayed a high level of expression (Fig. 1C). Only a few neurons in the axotomized DRGs did not express sodium channel mRNA I. Like control DRG neurons, nearly all axotomized DRG neurons expressed sodium channel mRNA II (Fig. 1F). While most axotomized DRG neurons expressed moderate levels of sodium channel mRNA II, some of neurons expressed high levels of this isoform. In contrast to control adult DRG neurons, axotomized DGR neurons clearly expressed sodium channel mRNA III (Fig. 1I). The levels of mRNA III expression in the axotomized DRG neurons ranged from low-to-moderately high.
E-l7 DRG
Since axotomy induced expression of sodium channel mRNA III in adult DRG neurons and this isoform has been shown to be expressed in high levels in the CNS during embryonic and neonatal stages of development (Beckh et al. 1989), we examined DRGs from E-17 fetuses to determine whether these neurons express sodium channel mRNA III.
In E-17 DRG neurons, sodium channel mRNA I is generally present at lower levels than in adult control DRG, with most neurons expressing mRNA I at low levels, though some neurons express a moderate level of this isoform (Fig. 1A). At this stage of development DRG neurons are substantially smaller than neurons in adult DRGs (cf. Fig. 1,A and B). Sodium channel mRNA II is expressed at moderate-to-high levels in E-17 DRG neurons (Fig. 1D). In contrast to DRG neurons from adult control DRGs, E-17 DRG neurons expressed sodium channel mRNA III, which is present in these cells at moderate-to-high levels (Fig. 1G).
Control experiments
Control experiments included omitting probe from the hybridization solution, substituting sense probes for anti-sense probes in the hybridization solution and pretreating tissue sections with RNAse A prior to hybridization. No specific labeling was observed in any of these controls (Fig. 2A) or with irrelevant probes (e.g., GFAP; Fig. 2B).
Fig. 2.
Control in situ hybridization sections. A : adult DRG sections were processed without a riboprobe in the hybridization solution. Faint background labeling is present in the DRG neurons. B: adult DRG neurons were hybridized with a riboprobe for GFAP mRNA, a marker for glial cells. Labeling of the neurons is negligible. Scale bar, 50 μm.
DISCUSSION
Electrophysiological observations on axotomized neurons have demonstrated abnormal dendritic electroresponsiveness that has been attributed to aberrant localization of sodium channels (Dodge and Cooley 1973; Sernagor et al. 1986; Titmus and Faber 1986). In the present study, we used in situ hybridization methods to ask whether different types of sodium channels are synthesized in DRG neurons following axotomy. We also asked whether there is a change in types of sodium channels expressed in embryonic versus adult DRG neurons. Our primary observations are that 1) mRNA for sodium channel α subunits I, II, and III are expressed in DRG neurons in embryonic rat, with mRNA I expressed at low-to-moderate levels, and mRNA II and III at high levels; 2) mRNA I and II are expressed at high levels, but mRNA III is not expressed at detectable levels, in mature DRG neurons in the adult rat; and 3) mRNA III is reexpressed, together with mRNA I and II that are expressed at relatively high levels, in adult DRG neurons following axotomy. These observations demonstrate altered expression of sodium channel mRNA in DRG neurons following axotomy, and suggest that, in at least some DRG neurons, there is a de-differentiation following axonal injury, with a reversion to an embryonic mode of sodium channel expression.
Our observation of a switch from expression of sodium channel mRNA III in embryonic DRG neurons, to its absence in mature DRG, is consistent with earlier hybridization blot analyses of DRG (Beckh et al. 1989; Beckh 1990) and with a developmental attenuation of mRNA III expression in other neurons observed by blot hybridization (Beckh et al. 1989; Beckh 1990) and in situ hybridization (Brysch et al. 1991; S. Yokoyama, J. A. Black, B. Ransom, H. Higashida, S. Waxman unpublished observations). Moreover, the increase in the level of expression of mRNA I in DRG neurons with increasing age is consistent with earlier results (Beckh et al. 1989; Beckh 1990).
Since only a subpopulation of neurons expressed each subtype of sodium channel mRNA, it is not possible to conclude, from the present in situ hybridization results, whether any given neuron expressed a single type of mRNA, or coexpressed several subtypes. Sensory axons are known to express several physiologically distinct sodium currents (Honmou et al. 1994; Jeftinija 1994; Neumcke and Stämpfli 1982; Stys et al. 1993). Moreover, DRG neurons can coexpress several types of sodium current (Caffrey et al. 1992; Honmou et al. 1994; Kostyuk et al. 1981; McLean et al. 1988; Roy and Narahashi 1992); thus, a one-to-one correlation between physiological characteristics of sodium currents and sodium channel mRNA subtype is not possible on the basis of the present results. Sodium channels distinct from types I, II, and III may also be present in DRG neurons. While it is tempting to speculate that type III mRNA may correspond to the kinetically slow, TTX-resistant sodium channels that are more abundantly present in immature DRG neurons (Roy and Narahashi 1992; Schwartz et al. 1990), it should be noted that TTX-resistant sodium currents are also present in some adult DRG neurons (Caffrey et al. 1992; Elliott and Elliott 1993; Yoshida et al. 1978). Unequivocal correlation between the sodium channel subtype(s) expressed in any given neuron, and the physiological characteristics of the sodium currents in that cell, will require correlative techniques such as electrophysiological analysis together with single-cell PCR, or patch-clamp recording with intracellular labeling carried out together with in situ hybridization using multiple labels for the entire spectrum of sodium channel mRNAs.
Even in the absence of a correlation between mRNA subtype and sodium current characteristics, our results are significant in indicating that, in at least some adult DRG neurons, there is a switch in the expression of sodium channel subtypes, with expression of type III sodium channel mRNA becoming apparent following axotomy. It is interesting, in this regard, that the expression of sodium channel mRNA III is triggered in rat brain neuronal cell lines (B35, B50, B65, B 103) in vitro by exposure to sodium butyrate, consistent with the idea that repressor or activator proteins may regulate transcription of sodium channel mRNA III (Baines et al. 1992).
While the regulatory mechanisms responsible for the differential expression of sodium channel subtypes in neurons are not yet understood, our results indicate that there is altered transcription of sodium channel mRNA in DRG neurons, with the expression of the type III message, after axotomy. An altered mode of sodium channel expression following axonal injury may be physiologically important, since it is now clear that abnormal dendritic excitability in axotomized neurons is due to sodium-dependent conductances (Sernagor et al. 1986; Titmus and Faber 1986). Different sodium channel characteristics, as well as an altered spatial distribution of sodium channels, may contribute to the electrophysiological changes that are seen in axotomized neurons.
Acknowledgments
This work was supported in part by the Medical Research Service, Department of Veterans Affairs, and by grants from the National Multiple Sclerosis Society and the National Institute of Neurological Disorders and Stroke. J. A. Black was supported in part by gifts from the Allen Charitable Trust and the Heumann Fund.
References
- Baines D, Mallon BS, Love S. Effects of sodium butyrate on the expression of sodium channels by neuronal cell lines derived from the rat CNS. Mol Brain Res. 1992;16:330–338. doi: 10.1016/0169-328x(92)90243-5. [DOI] [PubMed] [Google Scholar]
- Barron KD. Neuronal responses to axotomy: consequences and possibilities for rescue from permanent atrophy or cell death. In: Seil FJ, editor. Neural Regeneration and Transplantation. New York: Alan R. Liss; 1989. pp. 79–99. [Google Scholar]
- Beckh S. Differential expression of sodium channel mRNAs in rat peripheral nervous system and innervated tissues. FEBS Lett. 1990;262:317–322. 79–99. doi: 10.1016/0014-5793(90)80218-8. [DOI] [PubMed] [Google Scholar]
- Beckh S, Noda M, Lübbert H, Numa S. Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. EMBO J. 1989;8:361l–3616. doi: 10.1002/j.1460-2075.1989.tb08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhisitkul RB, Kocsis JD, Gordon TR, Waxman SG. Trophic influence of the distal nerve segment on GABAA receptor expression in axotomized adult sensory neurons. Exp Neural. 1990;109:273–278. doi: 10.1016/s0014-4886(05)80017-2. [DOI] [PubMed] [Google Scholar]
- Black JA, Yokoyama S, Higashida H, Ransom BR, Waxman SG. Sodium channel mRNA I, II, and III in the CNS: cell-specific expression. Mol Brain Res. 1994a;22:275–290. doi: 10.1016/0169-328x(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Black JA, Yokoyama S, Waxman SG, Oh Y, Zur KB, Sontheimer H, Higashida H, Ransom BR. Sodium channel mRNAs in cultured spinal cord astrocytes: in situ hybridization in identified cell types. Mol Brain Res. 1994b;23:235–245. doi: 10.1016/0169-328x(94)90230-5. [DOI] [PubMed] [Google Scholar]
- Brysch W, Creutzfeldt OD, Luno K, Schlingensiepen R, Schlingensiepen K-H. Regional and temporal expression of sodium channel messenger RNAs in the rat brain during development. Exp Brain Res. 1991;86:562–567. doi: 10.1007/BF00230529. [DOI] [PubMed] [Google Scholar]
- Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- Dodge FA, Jr, Cooley JW. Action potential of the motoneuron. IBM J Res Dev. 1973;17:219–229. [Google Scholar]
- Eccles JC, Libet B, Young RR. The behavior of chromatolysed motorneurons studied by intracellular recording. J Physiol Lond. 1958;143:11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol Lond. 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. II. Axotomized motoneurons and time course of recovery. J Neurophysiol. 1986;55:947–965. doi: 10.1152/jn.1986.55.5.947. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Motor-unit properties following cross-reinnervation of cat lateral gastrocnemius and soleus muscles with medial gastrocnemius nerve. II. Influence of muscle on motoneurons. J Neurophysiol. 1987;57:1227–1245. doi: 10.1152/jn.1987.57.4.1227. [DOI] [PubMed] [Google Scholar]
- Gallego R, Ivorra I, Morales A. Effects of central or peripheral axotomy on membrane properties of sensory neurones in the petrosal ganglion of the cat. J Physiol Lond. 1987;391:39–56. doi: 10.1113/jphysiol.1987.sp016724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstein B. The retina as a regenerating organ. In: Adler R, Farber DB, editors. The Retina: A Model for Cell Biology Studies, Part II. New York: Academic; 1986. pp. 275–335. [Google Scholar]
- Gustafsson B, Pinter MJ. Effects of axotomy on the distribution of passive electrical properties of cat motoneurones. J Physiol Lond. 1984;356:433–442. doi: 10.1113/jphysiol.1984.sp015474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. J Neurophysiol. 1994;71:1627–1638. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeftinija S. The role of tetrodotoxin-resistant sodium channels of small primary afferent fibers. Brain Res. 1994;639:125–134. doi: 10.1016/0006-8993(94)91772-8. [DOI] [PubMed] [Google Scholar]
- Kayano T, Noda M, Flockerzi V, Takahashi H, Numa S. Primary structure of rat brain sodium channel III deduced from the cDNA sequence. FEBS Lett. 1988;228:187–194. doi: 10.1016/0014-5793(88)80614-8. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Veselovsky NS, Tsyandryenko AY. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons. I. Sodium currents. Neuroscience. 1981;6:2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Kuno M, Llinas R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurons of the cat. J Physiol Lond. 1970;210:807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M, Miyata Y, Munoz-Martinez EJ. Differential reaction of fast and slow—motoneurones to axotomy. J Physiol Lond. 1974;240:725–739. doi: 10.1113/jphysiol.1974.sp010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford CJ, Scheffer JW, Jeffrey PL, Austin L. The in vitro synthesis of RNA within the rat nodose ganglion following vagotomy. J Neurochem. 1980;34:53l–539. doi: 10.1111/j.1471-4159.1980.tb11177.x. [DOI] [PubMed] [Google Scholar]
- Mclean MJ, Bennett PB, Thomas RM. Subtypes of dorsal root ganglion neurons based on different inward currents as measured by whole-cell voltage clamp. Mol Cell Biochem. 1988;80:95–107. doi: 10.1007/BF00231008. [DOI] [PubMed] [Google Scholar]
- Neumcke B, Stämpfli R. Sodium currents and sodium-current fluctuations in rat myclinated nerve fibres. J Physiol Lond. 1982;329:163–184. doi: 10.1113/jphysiol.1982.sp014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Ikeda T, Kayano T, Suzuki H, Tekeshima H, Kurasaki M, Takahashi H, Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature Lond. 1986;320:188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Omri G, Meiri H. Characterization of sodium currents in mammalian sensory neurons cultured in serum-free defined medium with and without nerve growth factor. J Membr Biol. 1990;115:12–29. doi: 10.1007/BF01869102. [DOI] [PubMed] [Google Scholar]
- Purves D, Nja A. Trophic maintenance of synaptic connections in autonomic ganglia. In: Cotman CW, editor. Neuronal Plasticity. New York: Raven Press; 1978. pp. 27–47. [Google Scholar]
- Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Palti Y, Meiri H. Structural and developmental differences between three types of Na channels in dorsal root ganglion cells of newborn rats. J Membr Biol. 1990;116:117–128. doi: 10.1007/BF01868670. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Yarom Y, Werman R. Sodium-dependent regenerative responses in dendrites of axotomized motoneurons in the cat. Proc Natl Acad Sci USA. 1986;83:7966–7970. doi: 10.1073/pnas.83.20.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapavolov AI, Grantyn AA. Suprasegmental synaptic influences on chromatolysed motor neurones. Biophysics. 1968;13:308–319. [Google Scholar]
- Stys PK, Ransom BR, Waxman SG. Non-inactivating, TTX-sensitive Na+ conductance in rat optic nerve axons. Proc Natl Acad Sci. 1993;90:6976–6980. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titmus MJ, Faber DS. Altered excitability of goldfish Mauthner cell following axotomy. II. Localization and ionic basis. J Neurophysiol. 1986;55:1440–1454. doi: 10.1152/jn.1986.55.6.1440. [DOI] [PubMed] [Google Scholar]
- Titmus MJ, Faber DS. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol. 1990;35:l–51. doi: 10.1016/0301-0082(90)90039-j. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Anderson MJ. Retrograde axon reaction following section of asynaptic nerve fibers. Cell Tissue Res. 1982;223:487–492. doi: 10.1007/BF00218470. [DOI] [PubMed] [Google Scholar]
- Wells MR. Changes of ornithine decarboxylase activity in dorsal root ganglion cells after axon injury: Possible relationship to alterations in neuronal chromatin. Exp Neurology. 1987;95:313–322. doi: 10.1016/0014-4886(87)90141-5. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Matsuda Y, Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J Neurophysiol. 1978;41:1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]


